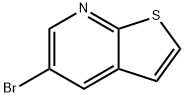
5-bromothieno[2,3-b]pyridine synthesis
- Product Name:5-bromothieno[2,3-b]pyridine
- CAS Number:21344-24-1
- Molecular formula:C7H4BrNS
- Molecular Weight:214.08
![Thieno[2,3-b]pyridin-5-amine (9CI)](/CAS/GIF/21344-28-5.gif)
21344-28-5
![5-bromothieno[2,3-b]pyridine](/CAS/20180703/GIF/21344-24-1.gif)
21344-24-1
General procedure for the synthesis of 5-bromothieno[2,3-b]pyridine from 5-aminothieno[2,3-b]pyridine: In Example 16E, 5-aminothieno[2,3-b]pyridine (1.35 g, 9.0 mmol) was reacted with isoamyl nitrite (Aldrich, 2.10 g, 18.0 mmol) and copper(II) bromide ( Aldrich, 4.03 g, 18.0 mmol) in acetonitrile (20 mL) at room temperature overnight. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride solution (20 mL) and subsequently extracted with ethyl acetate (3 x 50 mL). The organic phases were combined, washed with brine (2 x 30 mL) and concentrated. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane, v/v 80/20, Rf = 0.80) to afford 5-bromothieno[2,3-b]pyridine (1.03 g, 53.0% yield). The product was confirmed by 1H NMR (300 MHz, CDCl3): δ 8.61 (d, J=2.03 Hz, 1H), 8.21 (d, J=2.37 Hz, 1H), 7.58 (d, J=6.10 Hz, 1H), 7.22 (d, J=6.10 Hz, 1H). Mass spectra (DCI/NH3) showed m/z 214 (M+1)+ and 216 (M+1)+.
![Thieno[2,3-b]pyridin-5-amine (9CI)](/CAS/GIF/21344-28-5.gif)
21344-28-5
52 suppliers
$264.00/100mg
![5-bromothieno[2,3-b]pyridine](/CAS/20180703/GIF/21344-24-1.gif)
21344-24-1
47 suppliers
$60.00/1g
Yield: 53%
Reaction Conditions:
with copper(ll) bromide;isopentyl nitrite in acetonitrile at 20;
Steps:
16.E 5-Bromothieno[2,3-b]pyridine
Example 16E 5-Bromothieno[2,3-b]pyridine The product of Example 16D (1.35 g. 9.0 mmol) was treated with iso-amylnitrite (Aldrich. 2.10 g. 18.0 mmol) and CuBr2 (Aldrich, 4.03 g. 18.0 mmol) in MeCN (20 mL) at ambient temperature overnight. The reaction mixture was quenched with saturated NH4Cl (20 mL) and then extracted with EtOAc (3*50 mL). The combined extracts were washed with brine (2*30 mL) and concentrated. The residue was purified with chromatography (SiO2, EtOAc/hexane, v. 80/20. Rf=0.80) to give the title compound (1.03 g, yield, 53.0%). 1H NMR (300 MHz, CDCl3) 8 ppm 7.22 (d, J=6.10 Hz, 1H), 7.58 (d, J=6.10 Hz, 1H). 8.21 (d, J=2.37 Hz, 1H), 8.61 (d, J=2.0(3 Hz, 1H):MS (DCI/NH3) m/z 214 (M+1)+, 216 (M+1)+.
References:
Abbott Laboratories US2008/153860, 2008, A1 Location in patent:Page/Page column 25
![5-broMothieno[2,3-b]pyridine-2-carboxylic acid](/CAS/20150408/GIF/1242336-81-7.gif)
1242336-81-7
41 suppliers
$45.00/10mg
![5-bromothieno[2,3-b]pyridine](/CAS/20180703/GIF/21344-24-1.gif)
21344-24-1
47 suppliers
$60.00/1g
56267-50-6
134 suppliers
$16.00/250mg
2065-75-0
338 suppliers
$6.00/5g
![5-bromothieno[2,3-b]pyridine](/CAS/20180703/GIF/21344-24-1.gif)
21344-24-1
47 suppliers
$60.00/1g
![Acetamide, N-thieno[2,3-b]pyridin-5-yl-](/CAS/20210111/GIF/21344-35-4.gif)
21344-35-4
0 suppliers
inquiry
![5-bromothieno[2,3-b]pyridine](/CAS/20180703/GIF/21344-24-1.gif)
21344-24-1
47 suppliers
$60.00/1g