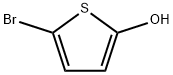
5-bromothiophen-2-ol synthesis
- Product Name:5-bromothiophen-2-ol
- CAS Number:1313392-39-0
- Molecular formula:C4H3BrOS
- Molecular Weight:179.04
162607-17-2

1313392-39-0
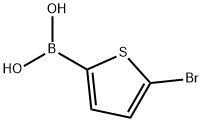
The general procedure for the synthesis of 2-hydroxy-5-bromothiophene from 5-bromothiophene-2-boronic acid was as follows: in a 25 mL reaction flask, pentafluorophenylhydrazine (0.35 mmol), 5-bromothiophene-2-boronic acid (0.5 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (1.0 mmol), water (1.5 mmol) and polyethylene glycol-600 (2.0 g). The mixture was stirred at 80 °C until the reaction was complete (monitored by TLC). Upon completion of the reaction, the reaction mixture was cooled to room temperature, followed by evaporation under reduced pressure to remove the solvent. Finally, the crude product was purified by column chromatography to afford the target compound 2-hydroxy-5-bromothiophene in 79% yield.

162607-17-2
201 suppliers
$8.00/250mg

1313392-39-0
33 suppliers
inquiry
Yield: 79%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene;pentafluorophenyl hydrazine in water at 80; for 12 h;
Steps:
31 Example 31 Compound 31:
A 25 mL reaction flask was charged with pentafluorophenylhydrazine (0.35 mmol)5-bromothiophene-2-boronic acid (0.5 mmol)1,8-diazabicyclo [5.4.0] undec-7-ene (1.0 mmol)Water (1.5 mmol)And polyethylene glycol-600 (2.0 g).The mixture was reacted at 80 ° C until the reaction was complete. The reaction mixture was cooled to room temperature and the solvent was evaporated under reduced pressure. The product was separated by column chromatography to yield a product in a yield of 79%.
References:
Nanjing Normal University;Han, Wei;Huang, Zheng;Yuan, Linxin;Gu, Wenchao;Shao, Ye CN103936538, 2017, B Location in patent:Paragraph 0073; 0093; 0094