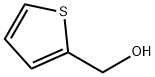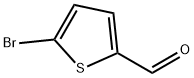
5-Bromothiophene-2-carbaldehyde synthesis
- Product Name:5-Bromothiophene-2-carbaldehyde
- CAS Number:4701-17-1
- Molecular formula:C5H3BrOS
- Molecular Weight:191.05
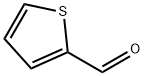
98-03-3

4701-17-1
2.1.1 Synthesis of 5-bromo-2-thiophenecarboxaldehyde Under nitrogen protection, 2-thiophenecarboxaldehyde (6.0 g, 53.5 mmol) was dissolved in anhydrous chloroform (125 mL). Subsequently, N-bromosuccinimide (10.4 g, 58.9 mmol) was slowly added and the reaction mixture was stirred for 12 h at room temperature. After completion of the reaction, the mixture was extracted with chloroform (3 × 50 mL), and the organic phases were combined and washed with deionized water (2 × 50 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography using chloroform as eluent to afford 5-bromo-2-thiophenecarboxaldehyde as a colorless oil (10.0 g, 98% yield). 1H NMR (400 MHz, CDCl3): δ 7.20 (d, 1H, J = 4.0 Hz), 7.53 (d, 1H, J = 8.0 Hz), 9.79 (s, 1H).

98-03-3
544 suppliers
$5.00/25g

4701-17-1
305 suppliers
$6.00/1g
Yield:4701-17-1 98%
Reaction Conditions:
with N-Bromosuccinimide in chloroform at 20;
Steps:
2.1.1 Synthesis processes of BDY-3T-BDY
2.1.1
Synthesis of 5-bromo-2-thiophenecarbaldehyde
To a solution of 2-thiophenecarboxaldehyde (6.0 g, 53.5 mol) in anhydrous CHCl3 (125 mL), N-bromosuccinimide (10.4 g, 58.9 mmol) was slowly added, and then the reaction mixture was stirred for overnight at room temperature.
The reaction mixture was extracted with CHCl3, and the organic phase was washed with deionized water, dried over Na2SO4, filtered, and evaporated to dryness to give the crude product.
Column chromatography was performed on silica gel using CHCl3 as the eluent, which affords the pure product as a colorless oil (10.0 g, 98%).
1H NMR (400 MHz, CDCl3): δ 7.20 (d, H, J = 4.0 Hz), 7.53 (d, H, J = 8.0 Hz), 9.79 (s, H).
References:
Tataroğlu;Al-Sehemi, Abdullah G.;Özdemir, Mehmet;Özdemir, Resul;Usta, Hakan;Al-Ghamdi, Ahmed A.;Farooq;Yakuphanoglu [Physica B: Condensed Matter,2017,vol. 519,p. 53 - 58]
765-58-2
208 suppliers
$6.00/1g

4701-17-1
305 suppliers
$6.00/1g
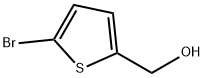
79387-71-6
144 suppliers
$24.00/1g

4701-17-1
305 suppliers
$6.00/1g
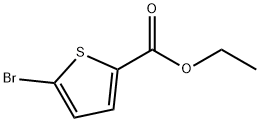
5751-83-7
156 suppliers
$6.00/1g

4701-17-1
305 suppliers
$6.00/1g