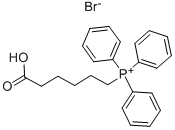
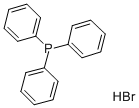
(5-Carboxypentyl)(triphenyl)phosphonium bromide synthesis
- Product Name:(5-Carboxypentyl)(triphenyl)phosphonium bromide
- CAS Number:50889-29-7
- Molecular formula:C24H26BrO2P
- Molecular Weight:457.34
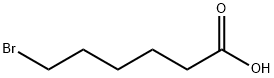
4224-70-8
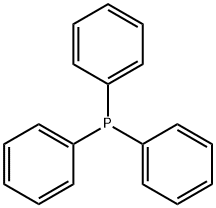
603-35-0

50889-29-7
General procedure for the synthesis of 5-carboxypentyltriphenylphosphonium bromide from 6-bromohexanoic acid and triphenylphosphine: 6-bromohexanoic acid (10.00 g, 51.27 mmol) and triphenylphosphonium (14.12 g, 53.83 mmol) were dissolved in freshly distilled acetonitrile (50 mL) and the reaction mixture refluxed for 48 h under vigorous stirring. Upon completion of the reaction, the solution was cooled to room temperature and precipitation of Wittig salt was induced by scraping the inner wall of the glass reactor. The resulting white solid product was collected by filtration and washed with ether to give the final 5-carboxypentyltriphenylphosphonium bromide in 98% (22.87 g) yield. The structure of the product was confirmed by 1H NMR (400 MHz, CD3OD): δ 1.62-1.72 (m, 6H), 2.29 (t, 2H), 3.40-3.47 (m, 2H), 7.76-7.91 (m, 15H).

4224-70-8
353 suppliers
$7.00/5g

50889-29-7
227 suppliers
$5.00/1g
Yield:50889-29-7 100%
Reaction Conditions:
with triphenylphosphine in toluene at 120; for 17 h;
Steps:
5.1 4.5.1 (6-Carboxyhexyl)triphenylphosphonium bromide (11)
In a 100 mL triple-necked round-bottom flask 5.01 g (19.1 mmol, 1.0 eq.) 6-bromohexanoic acid and 6.71 g (19.61 mmol, 1.0 eq.) triphenylphosphine were dissolved in 30 mL dry toluene. The reaction mixture was heated to 120 °C and stirred for 17 h. After cooling to room temperature, the product was collected by filtration and washed with toluene (2 * 20 mL) and Et2O (2 * 20 mL) before drying under reduced pressure. 11 was obtained as colorless powder in quantitative yield. m.p. = 201-205 °C; 1H NMR (300.36 MHz; CDCl3) δ = 9.12 (s, 1H), 7.74 (m, 15H), 3.60 (s, 2H), 2.34 (s, 2H), 1.64 (s, 6H); 13C NMR (75.53 MHz; CDCl3) δ = 175.9, 135.1, 133.7, 133.6, 130.7, 130.5, 34.27, 29.6, 24.0, 21.9, 22.2.
References:
Migglautsch, Anna K.;Willim, Melissa;Schweda, Bettina;Glieder, Anton;Breinbauer, Rolf;Winkler, Margit [Tetrahedron,2018,vol. 74,# 43,p. 6199 - 6204]
502-44-3
370 suppliers
$5.00/25g
6399-81-1
222 suppliers
$7.00/10g

50889-29-7
227 suppliers
$5.00/1g
1191-25-9
124 suppliers
$14.14/250mgs:

50889-29-7
227 suppliers
$5.00/1g

60633-18-3
0 suppliers
inquiry

50889-29-7
227 suppliers
$5.00/1g