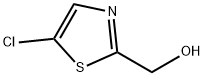
(5-chloro-1,3-thiazol-2-yl)methanol synthesis
- Product Name:(5-chloro-1,3-thiazol-2-yl)methanol
- CAS Number:50398-78-2
- Molecular formula:C4H4ClNOS
- Molecular Weight:149.6
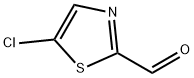
59129-52-1

50398-78-2
To a stirred methanolic solution of 5-chlorothiazole-2-carbaldehyde (0.5 g, 3.39 mmol) (6.78 mL) was slowly added sodium borohydride (0.128 g, 3.39 mmol) at room temperature. The reaction mixture was stirred continuously at room temperature for 30 min, followed by concentration under reduced pressure. The concentrated residue was dissolved in ethyl acetate (40 mL) and washed sequentially with deionized water (30 mL) and saturated saline (20 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give (5-chlorothiazol-2-yl)methanol as a white solid (yield: 101%, 511 mg). The product could be used for subsequent reactions without further purification.LC-MS (ESI+) analysis showed m/z = 150.1 ([M+H]+).1H NMR (400 MHz, CDCl3) δ 7.54 (s, 1H), 4.88 (d, J = 5.87 Hz, 2H), 2.57 (t, J = 6.06 Hz, 1H).

59129-52-1
65 suppliers
$45.00/25mg

50398-78-2
27 suppliers
$145.00/250MG
Yield:50398-78-2 511 mg
Reaction Conditions:
with methanol;sodium tetrahydroborate at 20; for 0.5 h;
Steps:
Sodium borohydride (0.128 g, 3.39 mmol) was added to a stirring solution of 5- chlorothiazole-2-carboxaldehyde (0.5 g, 3.39 mmol) in MeOH (6.78 mL) at RT. The mixture was stirred for 30 min at RT and then concentrated in vacuo. The residue was taken up in EtOAc (40 mL) and washed sequentially with water (30 mL) and brine (20 mL). The organic layer was then dried over Na2SO4, filtered and concentrated in vacuo to give (5-chlorothiazol-2-yl)methanol as a white solid that was used without further purification (405A, 511 mg, 101 %). LC/MS (ESI+) m/z = 150.1 (M+H)+. 1H NMR (400 MHz, chloroform-d) δ 7.54 (s, 1 H), 4.88 (d, J=5.87 Hz, 2H), 2.57 (t, J=6.06 Hz, 1 H).
References:
WO2016/22724,2016,A1 Location in patent:Page/Page column 270