
5-CHLORO-1-METHYL-1H-INDOLE-2-CARBOXYLIC ACID synthesis
- Product Name:5-CHLORO-1-METHYL-1H-INDOLE-2-CARBOXYLIC ACID
- CAS Number:59908-47-3
- Molecular formula:C10H8ClNO2
- Molecular Weight:209.63
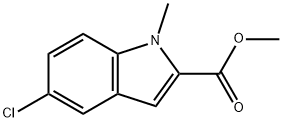
148356-79-0

59908-47-3
Step 2: Synthesis of 5-chloro-1-methyl-1H-indole-2-carboxylic acid (43.2) To a THF solution (10 mL) of methyl 5-chloro-1-methyl-1H-indole-2-carboxylate (43.1, 224 mg) was added an aqueous solution (2 mL) of LiOH (36 mg, 1.5 mmol). The reaction mixture was stirred at room temperature for 2 hours. Upon completion of the reaction, the solvent was removed under reduced pressure and the residue was dissolved in 1N aqueous hydrochloric acid solution (10 mL). The resulting suspension was allowed to stand for 10 minutes, then filtered through filter paper, collected as a solid and dried under vacuum to afford 163 mg (77.6% yield) of 5-chloro-1-methyl-1H-indole-2-carboxylic acid (43.2) as a beige powder.

148356-79-0
14 suppliers
$90.00/50mg

59908-47-3
77 suppliers
$45.00/10mg
Yield:59908-47-3 94%
Reaction Conditions:
with sodium hydroxide in ethanol at 50; for 0.5 h;
Steps:
1.2 Preparation of 5-chloro-1-methyl-1H-indole-2-carboxylic acid
77 mg (0.34 mmol) of the compound prepared in the above step 1 was dissolved in ethanol, 2M sodium hydroxide was added, and the mixture was stirred at 50 ° C for 30 minutes. After completion of the reaction, the reaction mixture was extracted with 50 ml of ethyl acetate, washed with 4N hydrochloric acid and water, and dried over anhydrous sodium sulfate.And concentrated under reduced pressure to obtain 67 mg (0.32 mmol) of the title compound as white solid in 94% yield.
References:
KR101725451,2017,B1 Location in patent:Paragraph 0294; 0301-0303
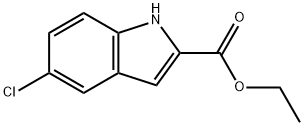
4792-67-0
251 suppliers
$10.00/5g

74-88-4
357 suppliers
$15.00/10g

59908-47-3
77 suppliers
$45.00/10mg

59908-53-1
8 suppliers
inquiry
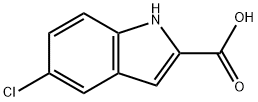
10517-21-2
291 suppliers
$22.00/1g

74-88-4
357 suppliers
$15.00/10g

59908-47-3
77 suppliers
$45.00/10mg
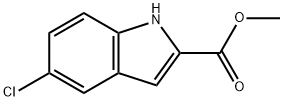
87802-11-7
66 suppliers
$16.00/250mg

59908-47-3
77 suppliers
$45.00/10mg
587-04-2
433 suppliers
$7.00/25g

59908-47-3
77 suppliers
$45.00/10mg