
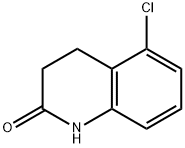
5-chloro-1-methyl-3,4-dihydroquinolin-2(1H)-one synthesis
- Product Name:5-chloro-1-methyl-3,4-dihydroquinolin-2(1H)-one
- CAS Number:1383842-66-7
- Molecular formula:C10H10ClNO
- Molecular Weight:195.65
72995-15-4
32 suppliers
inquiry

74-88-4
352 suppliers
$15.00/10g

1383842-66-7
13 suppliers
$45.00/2.5mg
Yield:1383842-66-7 82%
Reaction Conditions:
with sodium t-butanolate in tetrahydrofuran at 0 - 20;
Steps:
A-7.D ΓΡ1 5-Chloro- l-methyl-3,4-dihvdroquinolin-2-one
ΓΡ1 5-Chloro- l-methyl-3,4-dihvdroquinolin-2-one To a solution of 5-chloro-3,4-dihydro-lH-quinolin-2-one (16.0 g, 88.4 mmol), t-BuONa (16.0 g, 166.7 mmol) in THF (200 mL) was added methyl iodide (16.0 g, 140.4 mmol) drop wise at 0 °C. After the addition, the reaction mixture was slowly warmed up to room temperature and stirred overnight. It was then quenched with satd. aq. NaCl solution, extracted with diethyl ether (200 mL x 3), dried over anhy. Na2S04, and concentrated in vacuo. The residue was purified by silica gel flash chromatography to afford the desired title compound as a pale yellow solid (16.0 g, 82%). MS: 196.1 (M+H)+.
References:
WO2016/66662,2016,A1 Location in patent:Page/Page column 41

75002-98-1
88 suppliers
$13.00/250mg

1383842-66-7
13 suppliers
$45.00/2.5mg

1261455-37-1
9 suppliers
$200.00/100mg

1383842-66-7
13 suppliers
$45.00/2.5mg
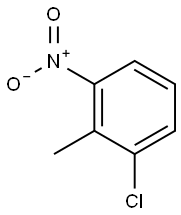
83-42-1
278 suppliers
$9.00/5g

1383842-66-7
13 suppliers
$45.00/2.5mg
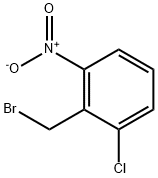
56433-01-3
76 suppliers
inquiry

1383842-66-7
13 suppliers
$45.00/2.5mg