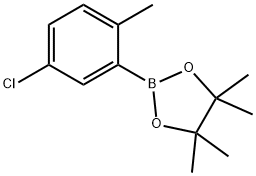
5-Chloro-2-Methylphenylboronic acid, pinacol ester synthesis
- Product Name:5-Chloro-2-Methylphenylboronic acid, pinacol ester
- CAS Number:1352426-91-5
- Molecular formula:C13H18BClO2
- Molecular Weight:252.54

73183-34-3
589 suppliers
$6.00/5g
95-79-4
266 suppliers
$13.00/5g

1352426-91-5
31 suppliers
$60.00/100mg
Yield:1352426-91-5 58%
Reaction Conditions:
Stage #1: 5-chloro-2-methyl-benzenaminewith tetrafluoroboric acid;sodium nitrite in water at 0; for 1.08333 h;
Stage #2: bis(pinacol)diboranewith triphenylphosphine in acetonitrile at 20; for 18 h;Inert atmosphere;Schlenk technique;
Steps:
Typical procedure for the borylation of arenediazonium salts
The arylamine (10 mmol) was dissolved in a mixture of 5 mL of distilled water and 3:4 mL of 50% tetrafluoroboric acid. After cooling the reaction mixture to 0 °C in an ice bath, sodium nitrite (0:69 g in 2 mL of distilled water ) was added dropwise within 5 min. The resulting mixture was stirred for 1 h, and the precipitate was collected by filtration and redissolved in the minimum amount of acetone. Diethyl ether was added until precipitation of the arenediazonium tetrafluoroborate, which was filtered, washed several times with diethylether, and dried under vacuum. An arenediazonium salt (1:5 mmol), bis(pinacolato)diborane(1 mmol) and PPh3 (2:0 eq.) were weighed in a 25 LSchlenk round bottom flask under nitrogen atmosphere. Then 3 mL of acetonitrile was added by syringe. The resulting solution was stirred at room temperature. The reaction progress was monitored by GC-MS. After the completion of the reaction,the solution was filtered though a short column of silica gel and the column washed with ethyl acetate. The filtrate was concentrated under reduced pressure to leave a crude product, which was purified by flash column chromatography on silica gel to afford the final products.
References:
Chen, Shuangshuang;Pan, Zhangjin;Wang, Yan [Zeitschrift fur Naturforschung - Section B Journal of Chemical Sciences,2014,vol. 69,# 9-10,p. 982 - 986]