
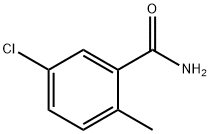
5-CHLORO-2-METHYLTHIOBENZAMIDE synthesis
- Product Name:5-CHLORO-2-METHYLTHIOBENZAMIDE
- CAS Number:1174906-84-3
- Molecular formula:C8H8ClNS
- Molecular Weight:185.67
1028252-11-0
15 suppliers
inquiry

1174906-84-3
7 suppliers
$60.00/1g
Yield:-
Reaction Conditions:
with Lawessons reagent in tetrahydrofuran at 23; for 1 h;
Steps:
4.2. Preparation of thioamides 2. General procedure
General procedure: Amides (1 mmol) and Lawesson's reagent (490 mg, 1.2 mmol) were added to dry THF (15 mL). The reaction mixture was stirred at room temperature for 1 h, or heated under reflux for 5 h in the case of the 4-nitro derivative. The solvent was evaporated under reduced pressure and the residue was partitioned between aq NaHCO3 (25 mL) and ethyl acetate (25 mL). The organic solvent was separated and dried over anhydrous MgSO4. The crude product was further purified by silica gel flash chromatography, using hexane-ethyl acetate (4:1), to yield the corresponding thioamides as yellow solids (42-60%). 4-Nitrothioamide (2ff),38 4-cyanothiobenzamide (2gg)39 and 3-cyanothiobenzamide (2hh),40 3-methoxythiobenzamide (2ii)41 have been previously reported.
References:
Mayhoub, Abdelrahman S.;Marler, Laura;Kondratyuk, Tamara P.;Park, Eun-Jung;Pezzuto, John M.;Cushman, Mark [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 1,p. 510 - 520] Location in patent:experimental part