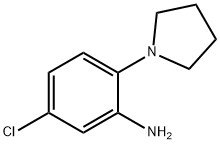
5-CHLORO-2-PYRROLIDIN-1-YLANILINE HYDROCHLORIDE synthesis
- Product Name:5-CHLORO-2-PYRROLIDIN-1-YLANILINE HYDROCHLORIDE
- CAS Number:59504-29-9
- Molecular formula:C10H13ClN2
- Molecular Weight:196.68

41173-36-8
12 suppliers
$23.30/250mg

59504-29-9
21 suppliers
$31.00/250mg
Yield:59504-29-9 96 mg
Reaction Conditions:
with ammonium chloride;zinc in ethanol; for 16 h;Inert atmosphere;Reflux;
Steps:
General Protocol D.
General procedure: N,N-Diethylbenzene-1,2-diamine (73). K2CO3 (1.47g, 12.0mmol) was added to 1-fluoro-2-nitrobenzene 8a (0.22mL, 2.13mmol) and N,N-diethylamine (0.99mL, 9.6mmol) in DMF (7.5mL) at 0°C. The reaction was stirred at 80°C for 4h. The reaction was diluted with EtOAc (20mL), washed with water (2×10mL) and then saturated brine (20mL). The organic layers were separated and dried with MgSO4 and concentrated in vacuo. The orange crude residue was added to a stirred solution of EtOH (20mL). The reaction atmosphere was evacuated and backfilled three times with nitrogen gas before the addition of zinc (1.4g, 21mmol) and ammonium chloride (1.14g, 21mmol). The reaction was heated to reflux for 16h before being filtered through Celite and washed with EtOH (30mL). The reaction mixture was concentrated to dryness and purified using silica chromatography eluting with 25% EtOAc/heptane to obtain 73 as a brown oil (107.5mg, 49%). 1H NMR (CDCl3, 300MHz) δ 7.02 (d, J 7.7Hz, 1H), 6.93 (t, J 7.6Hz, 1H), 6.79-6.66 (m, 2H), 2.95 (q, J 6.9Hz, 4H), 0.98 (t, J 7.0Hz, 6H). MS m/z (%) [M+H]+ 165 (100).
References:
Bailey, Brodie L.;Nguyen, William;Ngo, Anna;Goodman, Christopher D.;Gancheva, Maria R.;Favuzza, Paola;Sanz, Laura M.;Gamo, Francisco-Javier;Lowes, Kym N.;McFadden, Geoffrey I.;Wilson, Danny W.;Laleu, Beno?t;Brand, Stephen;Jackson, Paul F.;Cowman, Alan F.;Sleebs, Brad E. [Bioorganic Chemistry,2021,vol. 115,art. no. 105244]
345-18-6
202 suppliers
$7.00/5g

59504-29-9
21 suppliers
$31.00/250mg