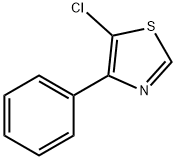
5-Chloro-4-phenylthiazole synthesis
- Product Name:5-Chloro-4-phenylthiazole
- CAS Number:1403935-14-7
- Molecular formula:C9H6ClNS
- Molecular Weight:195.67
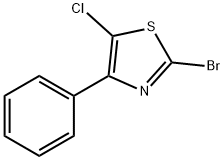
1403935-16-9
0 suppliers
inquiry

1403935-14-7
4 suppliers
inquiry
Yield:1403935-14-7 79%
Reaction Conditions:
with sodium tetrahydroborate;N,N,N,N,-tetramethylethylenediamine;palladium diacetate;triphenylphosphine in tetrahydrofuran at 25; for 9 h;Inert atmosphere;chemoselective reaction;
Steps:
General procedure for the hydrodehalogenation of halogenated heterocycles
General procedure: Pd(OAc)2-PPh3, Pd2(dba)3-tbpf, Pd2(dba)3-DavePhos Pd2(dba)3-P(t-Bu)3 Pd2(dba)3-XantPhos and Pd(OAc)2-XPhos. Anhydrous THF (13.2 mL) was degassed by bubbling argon for few minutes, then Pd(OAc)2 (7.2 mg, 0.033 mmol, 5 mol%) and PPh3 (17.7 mg, 1.132 mmol, 20 mol%) were added and the resulting mixture stirred at room temperature for 30 min. The halogenated heterocycle (0.66 mmol), TMEDA (0.130 g, 1.12 mmol, 1.7 equiv) and finally NaBH4 (42.4 mg, 1.12 mmol, 1.7 equiv) were introduced in sequence. The mixture was stirred at room temperature or heated at 65 °C under argon for the proper time. The residue was taken up in brine and extracted with ethyl acetate. The organic phase was separated, dried, the solvent was evaporated and the residue was purified by flash chromatography (mixtures of petroleum ether and ethyl acetate) to give pure hydrodehalogenated heterocycles
References:
Chelucci, Giorgio;Figus, Susanna [Journal of Molecular Catalysis A: Chemical,2014,vol. 393,p. 191 - 209]
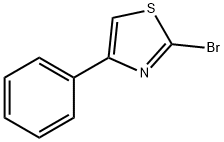
57516-16-2
76 suppliers
$31.00/250mg

1403935-14-7
4 suppliers
inquiry