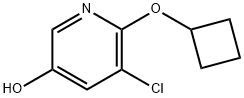
5-CHLORO-6-CYCLOBUTOXYPYRIDIN-3-OL synthesis
- Product Name:5-CHLORO-6-CYCLOBUTOXYPYRIDIN-3-OL
- CAS Number:1355066-45-3
- Molecular formula:C9H10ClNO2
- Molecular Weight:199.63
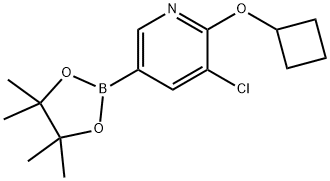
1352573-92-2
6 suppliers
inquiry

1355066-45-3
5 suppliers
inquiry
Yield:1355066-45-3 72%
Reaction Conditions:
with peracetic acid;acetic acid in water at 0; for 1 h;
Steps:
19
Acetic acid solution of peracetic acid (35%, 4.2 mL, 21.3 mmol) was added to a solution of 3-chloro-2-cyclobutoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine (Preparation 20, 4.4 g, 14.2 mmol) in glacial acetic acid (26 mL) and water (13 mL) at 0° C. The reaction mixture was stirred at 0° C. for 1 hour. Aqueous solution of sodium thiosulfate (0.5 M, 80 mL) was added and the mixture was stirred for 30 minutes at room temperature. The aqueous layer was extracted with EtOAc (3×40 mL). The combined organics were dried over magnesium sulfate, filtered and concentrated in vacuo. The crude residue was purified by silica gel chromatography eluting with 10% EtOAc in heptane to afford the title compound as a clear oil (2.05 g, 72%):1H NMR (400 MHz, CDCl3): δ 1.67 (m, 1H), 1.83 (m, 1H), 2.17 (m, 2H), 2.45 (m, 2H), 5.13 (m, 1H), 5.60 (s, 1H), 7.27 (m, 1H), 7.65 (m, 1H).LCMS Rt=2.44 minutes. MS m/z 202 [MH]+
References:
US2012/10183,2012,A1 Location in patent:Page/Page column 45
2402-77-9
656 suppliers
$5.00/5g

1355066-45-3
5 suppliers
inquiry

1288989-60-5
8 suppliers
inquiry

1355066-45-3
5 suppliers
inquiry