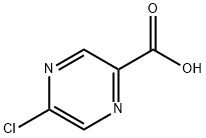
5-CHLORO-PYRAZINE-2-CARBOXYLIC ACID synthesis
- Product Name:5-CHLORO-PYRAZINE-2-CARBOXYLIC ACID
- CAS Number:36070-80-1
- Molecular formula:C5H3ClN2O2
- Molecular Weight:158.54
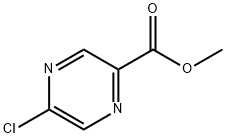
33332-25-1

36070-80-1
Example 29: Synthesis of 5-[2(R)-(3-chloro-4-methylsulfonyl-phenyl)-3-cyclopentylpropylamino]-pyrazine-2-carboxylic acid hydroxylamides [000197] General steps: 1. dissolve methyl 5-chloropyrazine-2-carboxylate (30.00 g, 0.17 mol) in tetrahydrofuran (87 mL). 2. a solution of potassium carbonate (72.08 g, 0.52 mol) in water (261 mL) was added to the above solution. 3. The reaction mixture was stirred at 25 °C for 42 h. 4. 4. Upon completion of the reaction, the reaction mixture was acidified to a pH of about 2 with concentrated hydrochloric acid. 5. The reaction mixture was diluted with saturated aqueous sodium chloride solution (300 mL). 6. Extracted successively with ethyl acetate (4 L total) until no product was detected in the aqueous layer. 7. The organic layers were combined, dried over sodium sulfate and filtered. 8. The filtrate was concentrated in vacuum to give 5-chloropyrazine-2-carboxylic acid (26.54 g, 96% yield) as an off-white solid. Physical properties: melting point 150-151°C; EH-HRMS m/e calculated value C5H3ClN2O2 (M+) 157.9883, measured value 157.9877.

33332-25-1
245 suppliers
$8.00/1g

36070-80-1
276 suppliers
$9.00/1g
Yield:36070-80-1 96%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran;water at 25; for 42 h;
Steps:
29
Example 29 5- [2 (R)- (3-CHLORO-4-METHANESULFONYL-PHENYL)-3-CYCLOPENTYL-PROPIONYLAMINO]- PYRAZINE-2-CARBOXYLIC acid HYDROXYAMIDE [000197] A solution of methyl 5-chloropyrazine-2-carboxylate (30.00 g, 0.17 mol) in tetrahydrofuran (87 mL) was treated with a solution of potassium carbonate (72.08 g, 0.52 mol) in water (261 mL). The resulting reaction mixture stirred at 25°C for 42 h. The reaction mixture was then acidified to a pH of about 2 with concentrated hydrochloric acid, diluted with a saturated aqueous sodium chloride solution (300 mL), and was continuously extracted with ethyl acetate (4L total) until no product was present in the aqueous layer. The combined organic layers were dried over sodium sulfate, filtered, and concentrated in vacuo to afford 5-chloro-pyrazine-2-carboxylic acid (26.54 g, 96%) as an off-white solid: mp 150-151°C ; EI-HRMS m/e calcd for CSH3CIN202 (M 157.9883, found 157.9877.
References:
F. HOFFMANN-LA ROCHE AG WO2004/52869, 2004, A1 Location in patent:Page 115 - 116
34604-60-9
209 suppliers
$15.00/1g

36070-80-1
276 suppliers
$9.00/1g
82619-63-4
30 suppliers
$45.00/10mg

36070-80-1
276 suppliers
$9.00/1g

34604-60-9
209 suppliers
$15.00/1g

36070-80-1
276 suppliers
$9.00/1g

33332-25-1
245 suppliers
$8.00/1g

111-27-3
547 suppliers
$5.00/25g
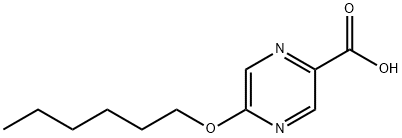
668972-67-6
0 suppliers
inquiry
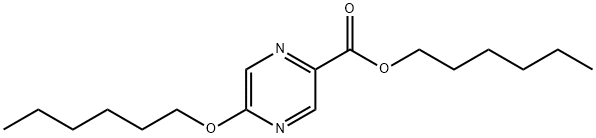
668972-68-7
0 suppliers
inquiry

36070-80-1
276 suppliers
$9.00/1g