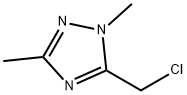
5-Chloromethyl-1,3-dimethyl-1H-[1,2,4]triazole synthesis
- Product Name:5-Chloromethyl-1,3-dimethyl-1H-[1,2,4]triazole
- CAS Number:84804-69-3
- Molecular formula:C5H8ClN3
- Molecular Weight:145.59

749931-96-2
0 suppliers
inquiry
![5-Chloromethyl-1,3-dimethyl-1H-[1,2,4]triazole](/CAS/20180906/GIF/84804-69-3.gif)
84804-69-3
19 suppliers
$45.00/10mg
Yield:84804-69-3 46%
Reaction Conditions:
Stage #1: C5H10ClN3Owith PPA at 120 - 130; for 4 h;
Stage #2: in water at 80; for 2 h;
Steps:
B.53.1
The title compound was prepared by a modification of a reported method (Kuebel, B. DE 3118258, Dec 2,1982) : A 2 L-round bottomed flask equipped with a magnetic stirrer was charged with acetamidine hydrochloride (94.5 g, 1.0 mol) and methanol (500 mL). Methylhydrazine (50.0 g, 1.1 mol) was added slowly via a dropping funnel over 30 min at room temperature under nitrogen blanket. After 2 d, the solvent was removed in vacuo, and the resulting residue triturated with ethyl acetate, filtered, and washed with ethyl acetate (3 x 400 mL). After drying the residue in a vacuum oven at 50 C, the crude intermediate amidrazone,/V- METHYLETHANEHYDRAZONAMIDE hydrochloride (109.8 g) was used directly in the next step. This intermediate (109.8 g) was suspended in DICHLOROMETHANE (400 mL), cooled to 0-5 C, and treated slowly with triethylamine (100.2 g) at this temperature. Chloroacetyl chloride (103.7 g, 1.02 mol) was added slowly over 30 min at 0-5 C via a dropping funnel. The reaction mixture was allowed to warm to room temperature and stirred for 18 h under nitrogen blanket. The solvent was then removed under reduced pressure, affording a residue (432.6 g) containing the N-CHLOROACETYL amidrazone intermediate. Polyphosphoric acid, PPA (400 G) was added to this material in a 2 L-3 necked flask, which was equipped with an overhead stirrer, a water condenser and a thermometer. The reaction mixture was stirred and heated at 120-130 C for 4 h. Upon cooling to 80 C, water (400 mL) was added slowly and stirring continued for an additional 2 h. Aqueous NAOH (100 G/150 mL) was used to adjust the pH from 3 to 9. The organic material was extracted with chloroform (4 x 1 L), and the resulting solution treated with activated charcoal, dried with Na2SO4, filtered, and evaporated carefully. The dark oily residue thus obtained was extracted with a mixture of ether (700 mL) and pentane (300 mL) to separate the product from insoluble impurities. The yellow supernatant was decanted and the solvent removed carefully in vacuo (30 C and-10 Torr), affording 60.0 g (46 % overall) of the title product as an oil (95% pure by NMR). 'H NMR (300 MHz, CDC13) 8 2.35 (s, 3H, C-CH3), 3.87 (s, 3H, N-CH3), and 4.62 (s, 2H, CH2). 13C NMR (300 MHz, CDC13) 8 14.05 (C-CH3), 34.37 (CH2), 35. 81 (N-CH3), 151.20 (Q-C) and 60.22 (Q-C). An NOE between CH2 and the N-CH3 was observed, consistent with the reported regioisomer. LC-MS (APCI) calcd for C5HSCLN3 : 145.04 ; found (M+H) 146.1 m/z (with chorine isotope pattern).
References:
WO2004/74270,2004,A2 Location in patent:Page 302