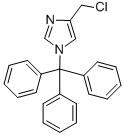
5-CHLOROMETHYL-1-METHYL-1H-IMIDAZOLE HCL synthesis
- Product Name:5-CHLOROMETHYL-1-METHYL-1H-IMIDAZOLE HCL
- CAS Number:17289-30-4
- Molecular formula:C5H7ClN2
- Molecular Weight:130.5755
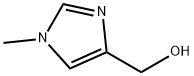
17289-25-7

17289-30-4
At 0 °C, (1-methyl-1H-imidazol-4-yl)methanol (5 g, 44.64 mmol) was dissolved in dichloromethane (10 mL) and thionyl chloride (50 mL) was added slowly and dropwise. The reaction mixture was stirred overnight at room temperature and then refluxed for 3 hours. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure. The residue was treated with diethyl ether and the resulting precipitate was filtered and dried to give 4-(chloromethyl)-1-methyl-1H-imidazole hydrochloride as a brown solid (4 g, 53.80% yield). The product was characterized by 1H NMR (300 MHz, DMSO-d6): δ 9.25 (s, 1H), 7.8 (s, 1H), 4.95 (s, 2H), 3.9 (s, 3H).

17289-25-7
111 suppliers
$19.00/100mg

17289-30-4
58 suppliers
$45.00/10mg
Yield: 53.81%
Reaction Conditions:
with thionyl chloride in dichloromethane at 0 - 20;Heating / reflux;
Steps:
2 Intermediate 2: 1-methyl-4-chloromethyl-imidazole hydrochloride
To a solution of intermediate 1 (5g, 44.64 [MMOL)] in CH2CI2 [(10 MI)] at [0°C] was added dropwise thionyl chloride (50 [ML)] and then the mixture was stirred at room temperature overnight and then under reflux for 3 hours and then concentrated under reduced pressure. The residue was treated with diethyl oxide and the resulting precipitate was filtered and dried. The title compound was obtained as a brown solid [(4G,] 53. 80%); 1HNMR (300 MHz, [DS-DMSO, PPM). S] : 9.25 (s , 1H), 7.8 (s, 1H), 4.95 (s, 2H), 3.9 (s, 3H).
References:
SMITHKLINE BEECHAM CORPORATION WO2004/13134, 2004, A2 Location in patent:Page 26