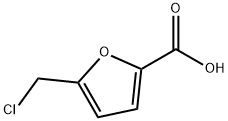
5-(chloroMethyl)furan-2-carboxylic acid synthesis
- Product Name:5-(chloroMethyl)furan-2-carboxylic acid
- CAS Number:39238-09-0
- Molecular formula:C6H5ClO3
- Molecular Weight:160.56
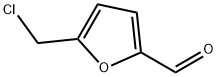
1623-88-7
93 suppliers
$60.00/25mg

39238-09-0
3 suppliers
inquiry
Yield:39238-09-0 92.8%
Reaction Conditions:
with hydrogenchloride;sodium chlorite in water;dimethyl sulfoxide;acetonitrile at 4 - 8;Concentration;
Steps:
2 Effect of Reactant Mole Ratio on Yield
[0269] This Example demonstrates the effect that the mole ratio of oxidant to reactant has on the oxidation of CMF to CMFA by NaC1O.[0270] A solution of CMF (5-chloromethylfurfural) (2.00 g, 0.0138 mol) in 50 mL of acetonitrile and 4.8 g (0.06 16 mol) of dimethyl sulfoxide was placed in an addition funnel. To a 250 mL round bottom flask was added 2.70 g of 81% sodium chlorite (0.0242 mol). Deionized water (50 mL) was added and the slurry was stirred until the solid dissolved. The solution was cooled in an ice water bath to <5 °C. To this was added 2.38 g of 37% hydrochloric acid (0.0242 mol), maintaining the mixture between 4 and 8 CC. The CMF solution was added drop-wise to the acidic sodium chlorite solution. The addition took about 44 mm. A TLC (silica gel, developed in 15% CH3OH/5% CHCI3/O.i% HCO2H, UV visualization) of the reaction about 1 h after starting the CMF addition showed very clean conversion of the CMF to CMFA (5-chloromethyl-2-furoic acid). The ice bath was removed. The reaction mixture was placed in the refrigerator overnight (<5 °C).O27i] Analysis of the reaction mixture by TLC the next morning showed a spot for the CMFA, and a faint spot for 5-hydroxymethylfuroic acid. The mixture was stirred and 3.44 g (27.3 mmol) of Na2SO3 was added. Stirring continued until the solid dissolved. This amount of sodium sulfite was sufficient to discharge the yellow color originally visible throughout the reaction mixture. The reaction mixture was poured into a 250 mL separatory funnel along with 25 mL of CH2C12. The mixture was shaken, allowed to settle, and then the bottom (aqueous) phase was drained off. The CH2C12/CH3CN phase was drained to a 250 mL Erlenmeyer flask. When a second 25 mL portion of CH2C12 was added to the aqueous phase which was returned to the separatory funnel, phase inversion occurred. This time the CH2C12 phase was on the bottom. After shaking, the bottom (organic) phase was drained off into the 250 mL Erlenmeyer flask. The remaining aqueous phase was extracted a third time with CH2C12. The combined organic phase was dried with 3 to 5 g of Mg504 and filtered to remove insoluble matter using a coarse sintered glass frit funnel. The solids were washed with about 10 mL of CH2C12. The colorless organic phase was analyzed by TLC. The organic phase contained mostly 5-chloromethyl-2-furoic acid and a trace of 5-hydroxymethylfuroic acid.[0272] The organic solution was concentrated on the rotary evaporator, bath temperature 40 °C. The partially solid, pale yellow residue weighed 4.24 g. This was re-dissolved in 25 mL of fresh CH2C12 and transferred into a 125 mL separatory funnel. The organics were washed with 20 mL of 1:1 saturated NaC1 brine/DI water. The CH2C12 solution was concentrated to dryness again on the rotary evaporator (bath temperature 30 °C). Some bumping occurred, so the solids were rinsed down with a minimal amount of CH2C12 and carefully concentrated to give a pale yellow, sticky solid, weight 3.31 g. A 72 mg sample was dissolved in CDC13ITMS and analyzed by ‘H- and ‘3C-NMR. ‘H-NMR peak assignments for CMFA (CDC13, 300 MHz): 6 10.90 (broad singlet, 1H), 7.22 (doublet (1=3.49 Hz), 1H), 6.53 (doublet (1=3.49 Hz), 1H), 4.6 15 (singlet, 2H). ‘3C-NMR peak assignments for CMFA (CDC13, 75 MHz): 6 161.6, 154.76, 144.6, 119.99, 111.63, 36.66. The material was 62.3 wt% CMFA, which is a 92.8% yield, higher than when a larger excess of NaC1O2 was employed for the oxidation as described in Example 2 above.
References:
WO2017/49211,2017,A1 Location in patent:Paragraph 0261; 0262; 0263; 0264-0268; 0269-0272; 0273-0284
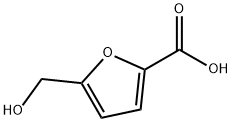
6338-41-6
208 suppliers
$5.00/100mg

39238-09-0
3 suppliers
inquiry
57-48-7
596 suppliers
$12.00/250G

39238-09-0
3 suppliers
inquiry
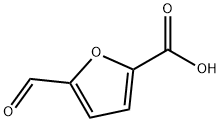
13529-17-4
163 suppliers
$16.00/100mg

39238-09-0
3 suppliers
inquiry