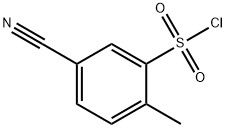
5-Cyano-2-methyl-benzenesulfonyl chloride synthesis
- Product Name:5-Cyano-2-methyl-benzenesulfonyl chloride
- CAS Number:372198-49-7
- Molecular formula:C8H6ClNO2S
- Molecular Weight:215.66
60710-80-7
129 suppliers
inquiry

372198-49-7
26 suppliers
inquiry
Yield:372198-49-7 25%
Reaction Conditions:
Stage #1: 5-cyano-2-methylanilinewith hydrogenchloride;sodium nitrite in water at 0; for 0.833333 h;
Stage #2: with copper(II) choride dihydrate;sulfur dioxide;acetic acid at 5; for 1.58333 h;
Steps:
4.1.3.10. 5-Cyano-N'-((5-cyanopyrazolo[1,5-a]pyridin-3-yl)methylene)-N,2-dimethylbenzenesulfonohydrazide (6j)
3-Amino-4-methylbenzonitrile (407 mg, 3.09 mmol) was suspended in concentrated HCl (3 mL) at 0 °C. A solution of NaNO2 (320 mg, 4.64 mmol) in water (1 mL) was added dropwise over 5 min, and the solution stirred for 45 min. Meanwhile, AcOH (3 mL) was saturated with SO2, then CuCl2·2H2O (158 mg, 0.93 mmol) was added and SO2 bubbled through for a further 5 min. The AcOH mixture was cooled to 5 °C, then the diazonium solution added over 5 min. The resulting mixture was stirred for a further 1.5 h, then the precipitate filtered off, washed with a little water and dried to leave 5-cyano-2-methylbenzenesulfonyl chloride as a yellow solid (167 mg, 25%). 1H NMR δ (400 MHz, CDCl3) 8.36 (d, J = 1.7 Hz, 1H), 7.87 (dd, J = 7.9, 1.7 Hz, 1H), 7.58 (d, J = 7.9 Hz, 1H), 2.88 (s, 3H). LC-MS (APCI-) 196 (M-Cl+O, 100%). Reaction of 2 (30 mg, 0.18 mmol) and the above sulfonyl chloride (45 mg, 0.21 mmol) using NaHCO3, after chromatography (eluting with CH2Cl2:MeOH 99.75:0.25) gave 6j as a yellow solid (32 mg, 48%).
References:
Kendall, Jackie D.;Giddens, Anna C.;Tsang, Kit Yee;Frédérick, Rapha?l;Marshall, Elaine S.;Singh, Ripudaman;Lill, Claire L.;Lee, Woo-Jeong;Kolekar, Sharada;Chao, Mindy;Malik, Alisha;Yu, Shuqiao;Chaussade, Claire;Buchanan, Christina;Rewcastle, Gordon W.;Baguley, Bruce C.;Flanagan, Jack U.;Jamieson, Stephen M.F.;Denny, William A.;Shepherd, Peter R. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 1,p. 58 - 68] Location in patent:experimental part