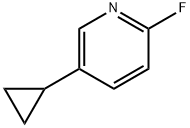
5-cyclopropyl-2-fluoropyridine synthesis
- Product Name:5-cyclopropyl-2-fluoropyridine
- CAS Number:1034467-80-5
- Molecular formula:C8H8FN
- Molecular Weight:137.15
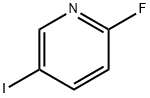
171197-80-1
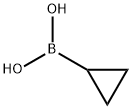
411235-57-9

1034467-80-5
General procedure for the synthesis of 5-cyclopropyl-2-fluoropyridine from 2-fluoro-5-iodopyridine and cyclopropylboronic acid: in a dry reaction flask, 2-fluoro-5-iodopyridine (1.12 g, 5 mmol), cyclopropylboronic acid (645 mg, 7.5 mmol), palladium acetate (56 mg, 0.25 mmol), potassium phosphate (3.2 g, 15 mmol) were added in sequence and a solvent mixture of toluene and water (20:1, 21 mL, by volume). The reaction mixture was heated at 100 °C for 4 h with stirring. After completion of the reaction, the reaction mixture was diluted with a solvent mixture of chloroform and isopropanol (3:1, 100 mL, by volume). The organic phase was washed sequentially with saturated aqueous sodium chloride solution and deionized water. The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give a brown oily crude product. Purification by column chromatography (eluent: hexane solution of 20% ethyl acetate) afforded 5-cyclopropyl-2-fluoropyridine (430 mg, 63% yield) as a light yellow oil. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.99 (d, J = 3 Hz, 1H), 7.39 (td, J = 3, 5 Hz, 1H), 6.79 (dd, J = 3, 8 Hz, 1H), 0.96-1.02 (m, 2H), 0.63-0.69 (m, 2H).
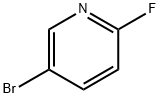
766-11-0
452 suppliers
$6.00/5g

411235-57-9
427 suppliers
$6.00/1g

1034467-80-5
44 suppliers
$45.00/10mg
Yield: 72%
Reaction Conditions:
with palladium diacetate;tricyclohexylphosphine in 1,2-dimethoxyethane;water at 80;
Steps:
16 5-cyclopropyl-2-fluoropyridine
A mixture of 5-bromo-2-fluoropyridine (435 mg, 2.5 mmol), cyclopropylboronic acid (260 mg, 3.0 mmol), potassium phosphate (1.27 g, 6.0 mmol), palladium (II) acetate (56 mg, 0.6 mmol) and tricyclohexyl phosphine (340 mg, 1.2 mmol) in 1,2- dimethoxyethane/water (10.0 mL/2.0 mL) was stirred at 80 °C overnight. The mixture was cooled and concentrated to dryness. The residue was purified by flash chromatography (petroleum ether / ethyl acetate = 3/1) to afford 5-cyclopropyl-2- fluoropyridine (246 mg, 1.8 mmol, 72% yield) as yellow solid. LCMS: m/z = 138.1 [M+H]+, retention time = 2.25 min (Method A).
References:
AKEBIA THERAPEUTICS, INC. WO2021/188944, 2021, A1 Location in patent:Paragraph 0432-0433

171197-80-1
299 suppliers
$20.00/1g

411235-57-9
427 suppliers
$6.00/1g

1034467-80-5
44 suppliers
$45.00/10mg