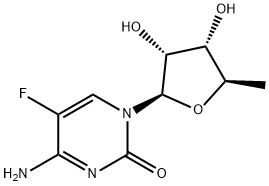
5'-Deoxy-5-fluorocytidine synthesis
- Product Name:5'-Deoxy-5-fluorocytidine
- CAS Number:66335-38-4
- Molecular formula:C9H12FN3O4
- Molecular Weight:245.21
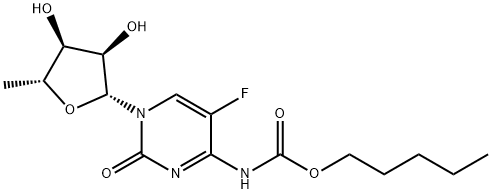
154361-50-9

66335-38-4
1. Pentyl (1-((2R,3R,4S,5R)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate (capecitabine, 4.5 g, 12.5 mmol) was suspended with potassium carbonate (8.8 g, 63.77 mmol) in methanol (250 mL) and N,N- dimethylformamide (DMF, 10 mL). The mixture was heated to reflux for 24 hours. Upon completion of the reaction, the reaction solution was cooled and concentrated to dryness under reduced pressure at below 45 °C. The residue was dissolved in hot methanol, filtered and washed with hot methanol. The filtrate was pre-adsorbed on silica gel and purified by fast column chromatography using 50% methanol/ethyl acetate as eluent to afford 1-[3,4-dihydroxy-5-methyltetrahydrofuran-2-yl]-4-amino-1H-pyrimidin-2-one (3.0 g, 90% yield). 2. The above product (3.0 g) was suspended in chloroform (125 mL) and heated to 50 °C. The product (3.0 g) was added to the mixture. Acetic acid (2 mL, 34 mmol) and acetyl chloride (20 mL, 206 mmol) were added sequentially and stirred at 50 °C for 7 hours, followed by continued stirring at 20 °C for 72 hours. At the end of the reaction, ether (100 mL) was added, the solid was collected by filtration and washed with ether to afford 1-[3,4-diacetoxy-5-methyltetrahydrofuran-2-yl]-4-amino-1H-pyrimidin-2-one hydrochloride (4.0 g, 90% yield). 3. The above hydrochloride (2.07 g, 5.7 mmol) was dissolved in pyridine (1.4 mL, 17.4 mmol) and dichloromethane (DCM, 15 mL) with 5-nitrothiophen-2-ylmethanol (1.47 g, 9.3 mmol). A 2M toluene solution of phosgene (3.6 mL, 7.2 mmol) was slowly added at 0 °C and stirred for 2.5 h. After 2.5 h, the same amount of phosgene solution was added again and stirring was continued for 2 h at 0 °C, followed by 18 h of refrigeration. The reaction solution was partitioned with ethyl acetate and brine, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, dried and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with 2% methanol/DCM as eluent to give an off-white foamy product (400 mg, 14% yield). Thin layer chromatography (TLC) showed Rf=0.45 (2% methanol/ethyl acetate as unfolding agent). Liquid chromatography-mass spectrometry (LC-MS) analysis showed a retention time of 4.68 min (TFA 20-50% gradient) and a mass m/z of 201/159/143. nuclear magnetic resonance hydrogen spectra (1H NMR, 500 MHz, DMSO-d6) δ 11.20 (1H, broad single peak, NH), 8.3 (1H, broad peak, NCH=CF). 8.05 (1H, single peak, HarH), 7.30 (1H, single peak, HarH), 5.80 (1H, single peak, NCHO), 5.42 (3H, multiple peaks, HarCH2OCONH, CHOAc), 5.15 (1H, single peak, CHOAc), 4.05 (1H, multiple peaks, OCHCH3), 3.45 (2H, multiple peak, 2×CHOAc), 2.48 (3H, single peak, OAc), 2.05 (3H, single peak, OAc), 1.37 (3H, single peak, OCHCH3) ppm.

154361-50-9
840 suppliers
$5.00/1g

66335-38-4
361 suppliers
$8.00/250mg
Yield:66335-38-4 90%
Reaction Conditions:
with potassium carbonate in methanol;N,N-dimethyl-formamide for 24 h;Heating / reflux;
Steps:
9
Capecitabine (5'-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine, 4.5 g, 12.5 mmol) was suspended in methanol (250 mL) and DMF (10 mL) together with potassium carbonate (8.8 g, 63.77 mmol) and the solution heated under reflux for 24 h. The solution was then cooled and evaporated to dryness (below 45 °C). The residue was redissolved in hot methanol, filtered and washed with hot methanol. The filtrate was preabsorbed onto silica gel and puified by flash chromatography, eluting with 50 % methanol / ethyl acetate to give 3.0 g (90 %) of l-[3,4-dihydroxy-5-methyl- tetrahydrofuran-2-yl]-4-amino-lH-pyrimidin-2-one. This material was suspended in chloroform (125 mL) and the solution heated to 50 0C. Acetic acid (2 mL, 34 mmol) was added and after 10 minutes at 50 0C, acetyl chloride (20 mL, 206 mmol) was added. The suspension was stirred at 50 0C for 7 h and then at 20 °C for 72 h. Ether (100 mL) was added and the solid filtered and washed with ether to give 4.0 g (90 %) l-[3,4-Diacetoxy-5-methyl-tetrahydrofuran-2-yl]-4-amino-lH-pyrimidin-2-one hydrochloride. This material (2.07g, 5.7 mmol) together with 5-nitrothien-2- ylmethanol (1.47 g, 9.3 mmol), was dissolved in pyridine (1.4 mL, 17.4 mmol) and DCM (15 mL). Phosgene solution (3.6 mL of a 2M solution in toluene, 7.2 mmol) was slowly added to the above cooled (O0C) solution, the solution was stirred at O0C for 2.5 h and then a further 3.6 mL of phosgene solution added and the solution stirred at O0C for a further 2h and refrigerated for 18 h. The solution was partitioned (ethyl EPO
References:
ANGIOGENE PHARMACEUTICALS LIMITED;THE GRAY LABORATORY CANCER RESEARCH TRUST WO2006/32921, 2006, A1 Location in patent:Page/Page column 37-38
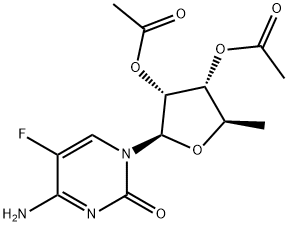
161599-46-8
436 suppliers
$6.00/250mg

66335-38-4
361 suppliers
$8.00/250mg
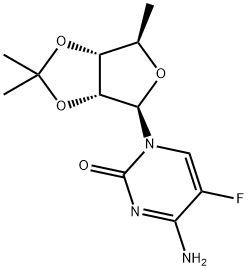
66335-37-3
48 suppliers
inquiry

66335-38-4
361 suppliers
$8.00/250mg