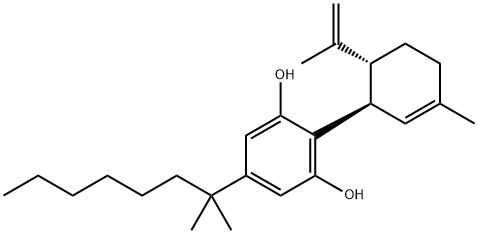
(-)-5'-DMH-CBD synthesis
- Product Name:(-)-5'-DMH-CBD
- CAS Number:97452-63-6
- Molecular formula:C25H38O2
- Molecular Weight:370.57
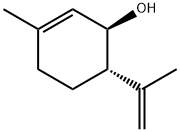
65733-30-4
0 suppliers
inquiry
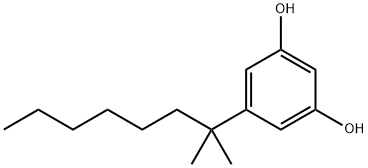
56469-10-4
154 suppliers
$20.00/250mg

97452-63-6
14 suppliers
inquiry
Yield:97452-63-6 80%
Reaction Conditions:
Stage #1: (+)-p-mentha-1,8-diene-3-olwith aluminum oxide;boron trifluoride diethyl etherate in dichloromethane at 20; for 0.25 h;Inert atmosphere;
Stage #2: 5-(1',1'-dimethyl-n-heptyl)-1,3-dihydroxybenzene in dichloromethane;Inert atmosphere;
Steps:
3.A Step A
Basic aluminium oxide (15.6 g) was added to dry dichloromethane (150 ml). To this suspension BF3 Et2 (2.3 ml) was added under nitrogen. The mixture was stirred for 15 min at room temperature under N2 atmosphere. To the solution was added (+)-p-mentha-1,8-diene-3-ol ((+)-isopiperitenol) 10 (950 mg, 6.25 mmol) and dimethylhepty resorcinol 11 (1 .45 g,6.25 mmol) in dry dichloromethane (50 ml) and the reaction mixture was quenched within 10 sec with 10% aqueous solution of sodium bicarbonate (50 ml). The organic layer was separated and the aqueous layer was further extracted with dichloromethane. The combined dichloromethane solution was extracted with water, brine, dried on Na2SO4 and evaporated to give an red oil. This oil was purified by silica gel column chromatography, using petroleum ether and ether as an eluent to obtain Compound 12 (1.86g, 80%). 1 H-NMR (CDCl3, 300 MHz): δ 6.250-6.358 (br, 2H, ArH), 5.90-6.050 (br, 1 H, OH), 5.560 (s, 1 H), 4.656 (s, 1 H), 4.545 (s, 1 H), 4.556 (s, 1 H), 3.850 (br, 1 H), 2.050-2.300 (m, 2H), 1 .794 (s, 3H), 1 .635 (s, 3H), 1 .454-1 .505 (m, 2H), 1 .234-1 .335 (m, 3H), 1 .208 (br s, 12H), 0.950-1 .050 (br, 2H), 0.832 (t, J = 7.5 Hz, 3H); GC- MS m/z: 370, 302, 287, 249, 217, 202, 187 [a]20D = -65.1° in CHCI3
References:
WO2017/8136,2017,A2 Location in patent:Page/Page column 39; 40
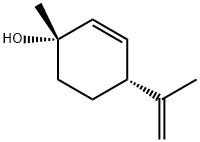
22972-51-6
231 suppliers
$18.00/1g

56469-10-4
154 suppliers
$20.00/250mg

97452-63-6
14 suppliers
inquiry