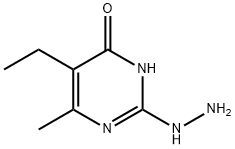
5-Ethyl-2-hydrazino-6-methylpyrimidin-4(3H)-one synthesis
- Product Name:5-Ethyl-2-hydrazino-6-methylpyrimidin-4(3H)-one
- CAS Number:65004-40-2
- Molecular formula:C7H12N4O
- Molecular Weight:168.2
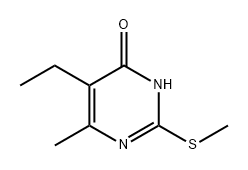
54855-82-2
0 suppliers
inquiry

65004-40-2
7 suppliers
inquiry
Yield:65004-40-2 62%
Reaction Conditions:
with hydrazine hydrate in ethanol at 100; for 12 h;
Steps:
Step 3
5-Ethyl-6-methyl-2-(methylthio)pyrimidin-4(3H)-one (619 mg, 3.3 mmol), hydrazine monohydrate (0.24 mL, 5 mmol), and EtOH (4 mL) were combined in a microwave tube and heated at 100 °C for 12 h. Then, the solution was filtered and the solid was washed with EtOH and dried overnight. The filtrate was condensed to give purple oil, and EtOH and EtOAc were added to precipitate out any remaining product. Some solid formed and was filtered and dried overnight. The samples were combined to give 5-ethyl-2-hydrazineyl-6-methylpyrimidin- 4(3H)-one as a light purple solid (352 mg, 62% yield). 1H NMR (400 MHz, DMSO-^e) d 2.26 (q, J= 8.0 Hz, 2H), 2.05 (s, 3H), 0.91 (t, J= 8.0 Hz, 3H). ESI-MS (m/z): 169.1 [M+H]+.
References:
WO2021/77102,2021,A1 Location in patent:Page/Page column 60-61
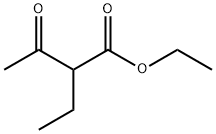
607-97-6
220 suppliers
$10.00/1g

65004-40-2
7 suppliers
inquiry

39083-15-3
18 suppliers
$168.30/5MG

65004-40-2
7 suppliers
inquiry