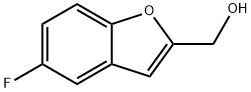
(5-FLUORO-1-BENZOFURAN-2-YL)METHANOL synthesis
- Product Name:(5-FLUORO-1-BENZOFURAN-2-YL)METHANOL
- CAS Number:276235-91-7
- Molecular formula:C9H7FO2
- Molecular Weight:166.15
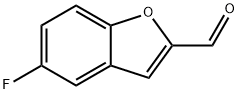
140382-35-0
16 suppliers
inquiry

276235-91-7
20 suppliers
$500.00/500mg
Yield:276235-91-7 91%
Reaction Conditions:
with sodium tetrahydroborate;ethanol at 0 - 20;
Steps:
(5-fluorobenzofuran-2-yl)methanol (14); Compound 13 (180 mg, 1.10 mmol) was dissolved in EtOH (12 mL). NaBH4 (45 mg, 1.21 mmol) was added portionwise at 0 °C, with vigorous stirring. The suspension was stirred at 0 °C for 15 min and then at room temperature for 1.5 h. Solvents were evaporated off in-vacuo. The crude residue was taken up in EtOAc and washed with water, brine and dried (MgSO4). The solvent was evaporated off in vacuo. The residue was adsorbed on silica gel and purified by flash chromatography, eluting with hexane/EtOAc (3:1 ) to give 14 (150 mg, 91%) as a white solid. 1H NMR (500MHz, CDCI3): δ: 7.36 (1 H, d, J = 8.35 Hz, H-7), 7.19 (1 H, d, J = 7.80, H-4), 7.00 (1 H, t, J = 8.75 H-6), 6.60 (1 H, s, H-3), 4.75 (2H, s, CH2).
References:
WO2010/125350,2010,A1 Location in patent:Page/Page column 40-41
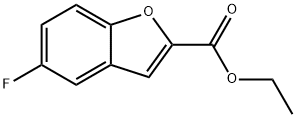
93849-31-1
27 suppliers
inquiry

276235-91-7
20 suppliers
$500.00/500mg
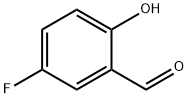
347-54-6
284 suppliers
$14.00/1g

276235-91-7
20 suppliers
$500.00/500mg
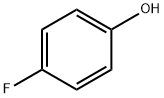
371-41-5
534 suppliers
$8.00/25g

276235-91-7
20 suppliers
$500.00/500mg