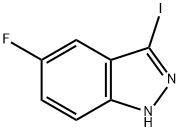
5-Fluoro-3-iodo-1H-indazole synthesis
- Product Name:5-Fluoro-3-iodo-1H-indazole
- CAS Number:858629-06-8
- Molecular formula:C7H4FIN2
- Molecular Weight:262.02
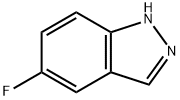
348-26-5

858629-06-8
5-Fluoro-1H-indazole (2.0 g, 14.0 mmol) was used as raw material and dissolved in DIVIF (50 mL). To this solution, iodine (7.46 g, 28.0 mmol) and potassium hydroxide (2.4 g, 42 mmol) were added sequentially at 0 °C. The reaction mixture was stirred at 0 °C for 0.5 h and then gradually warmed up to room temperature. The reaction process was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was filtered and the filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate=3/1) to afford 5-fluoro-3-iodo-1H-indazole (3.7 g, yield: 96.1%) as a white solid. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6): δ=13.64 (s, 1H), 7.63 (dd, J=4.8,4.4 Hz, 1H), 7.38-7.30 (m, 1H), 7.20 (dd, J=6.4,2.4 Hz, 1H).

348-26-5
167 suppliers
$10.00/250mg

858629-06-8
94 suppliers
$11.00/250mg
Yield:858629-06-8 96.1%
Reaction Conditions:
with iodine;potassium hydroxide in N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
616.1 Step 1
To a solution of 5-fluoro-1H-indazole (2.0 g, 14.0 mmol) in DIVIF (50 mL) was added ‘2 (7.46 g, 28.0 mmol) and KOH (2.4 g, 42 mmol), the mixture was stirred at room temperature for 0.5 hr. The reaction was monitored by TLC. After completion, the mixture was filtered, the filtrate was concentrated in vacuum to give a residue, which was purified by a silica gel column (PE/EA = 3/1) to afford 5-fluoro-3-iodo-1H-indazole (3.7 g, yield: 96.1%) as a white solid. ‘H NIVIR (400 IVIHz, DMSO-d6): ? = 13.64 (s, 1H), 7.63 (dd, J= 4.8, 4.4 Hz, 1H), 7.38-7.30 (m, 1H), 7.20 (dd, J=6.4,2.4Hz, 1H).
References:
WO2018/132372,2018,A1 Location in patent:Paragraph 01229