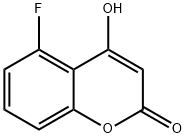
5-Fluoro-4-hydroxy-2H-chromen-2-one synthesis
- Product Name:5-Fluoro-4-hydroxy-2H-chromen-2-one
- CAS Number:799262-09-2
- Molecular formula:C9H5FO3
- Molecular Weight:180.13
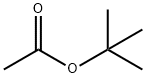
540-88-5
390 suppliers
$21.00/25mL
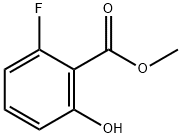
72373-81-0
103 suppliers
$25.00/250mg

799262-09-2
11 suppliers
inquiry
Yield: 12.5%
Reaction Conditions:
Stage #1:acetic acid tert-butyl ester with lithium diisopropyl amide;N-benzylbenzamide in tetrahydrofuran;n-heptane at -60; for 0.833333 h;
Stage #2:Methyl 2-fluoro-6-hydroxybenzoate in tetrahydrofuran;n-heptane at -60 - 20;
Steps:
B1
Method B1; 5-FLUORO-4-HYDROXYCOUMARIN; A catalytic amount of N-BENZYL-BENZAMIDE (Aldrich) and dry THF (Aldrich) (20 mL) were put into a three neck flask (inert atmosphere). To the cooled solution (-60 °C), 2.0 M solution of LDA (Aldrich) (10 mmole) in heptane (5 mL) was added. Subsequently, a solution of TERT-BUTYL acetate (Aldrich) (1.3 ML, 10 mmole) dissolved in dry THF (4 mL) was slowly added to the reaction mixture drop by drop and the stirring was continued for 50 minutes at the temperature OF-60 °C, whereat the colour of solution changed from red-brown to yellow. A solution obtained by dissolving methyl-6-fluorosalicylate (474 mg; 2.4 mmole) in dry THF (10 mL) was added slowly to the carbanion solution and the stirring was continued overnight, whereat the temperature of the reaction mixture reached room temperature. Ethyl acetate (50 mL) and a saturated solution of ammonium chloride (50 mL) were added to the mixture, the layers were separated and then the organic layer was washed with a saturated sodium chloride solution (2 x 50 mL) and dried with sodium sulfate. The solvent was removed by evaporation under reduced pressure. The remaining brown oily product was mixed with trifluoroacetic acid (3 mL) and the obtained mixture was stirred for 4 hours at room temperature and diluted by a hexane: ether (2: 1) solvent mixture (30 mL). A light brown precipitate was precipitated, which by purification on a silica gel column (12 g) using the solvent system chloroform: methanol: acetic acid (60: 10 : 1) yielded 54 mg (12.5 %) of light brown amorphous 5-fluoro-4-hydroxycoumarin. 1H-NMR (300 MHz, DMSO-d6) I5/PPM : 5.66 (s, 1H); 7.09 (ddd, J = 1.0 Hz; J = 8.4 Hz, J = 11.2 Hz, 1H); 7.17 (m, 1H); 7.64 (dt, J = 6.7 Hz, J = 8.4 Hz, 1H) ; 11.00 (bs, 1H) ; 3C-NMR (75.4 MHz, DMSO-d6) RPPM : 93.2 ; 106.7 (d, J = 12.7 Hz) ; 112.0 (d, J = 11.5 Hz); 130.6 (d, J = 3.5 Hz); 133.7 (d, J = 10.6 Hz); 156.2 (d, J = 4.5 Hz); 159.9 (d, J= 263. 0 Hz); 161.6 ; 165.7.
References:
PLIVA-ISTRAZIVACKI INSTITUT D.O.O. WO2005/10006, 2005, A1 Location in patent:Page/Page column 64