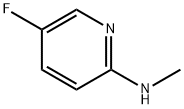
(5-Fluoro-pyridin-2-yl)-Methyl-aMine synthesis
- Product Name:(5-Fluoro-pyridin-2-yl)-Methyl-aMine
- CAS Number:868636-72-0
- Molecular formula:C6H7FN2
- Molecular Weight:126.13

74-88-4

868636-72-0
Step 1. Synthesis of 5-fluoro-N-methylpyridin-2-amine: A suspension of sodium hydride (60% dispersed in mineral oil, 117.8 mg, 4.91 mmol) was made in dry tetrahydrofuran (25 mL). Subsequently, a solution of 5-fluoropyridin-2-amine (500 mg, 4.46 mmol) in tetrahydrofuran (25 mL) was added dropwise to this suspension. The reaction mixture was stirred at room temperature and maintained at 40 °C for 30 min. After that, the reaction system was cooled to -40 °C and methyl iodide (696.9 mg, 4.91 mmol) was slowly added. After addition, the reaction mixture was returned to room temperature and stirred overnight. Upon completion of the reaction, the reaction was quenched by the addition of water and the mixture was extracted with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting crude product was purified by fast column chromatography on silica gel with hexane/ethyl acetate (4:1, v/v) as eluent to give 129 mg of the title compound in 23% yield. The product was characterized by 1H-NMR (CDCl3): δ 7.97 (1H, d, J = 2.6 Hz), 7.28-7.17 (1H, m), 6.34 (1H, dd, J = 3.5, 9.1 Hz), 2.90 (3H, d, J = 5.1 Hz).

74-88-4
357 suppliers
$15.00/10g

868636-72-0
47 suppliers
$110.00/100mg
Yield:868636-72-0 23%
Reaction Conditions:
Stage #1: 2-amino-5-fluoropyridinewith sodium hydride in tetrahydrofuran at 40; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran at -40 - 20;
Steps:
25.1
STEP 1. 5-Fluoro-N-methylpyridin-2-amine; To a suspension of sodium hydride (60% dispersion in mineral oil, 117.8 mg, 4.91 mmol) in tetrahydrofuran (25 ml) was added a solution of 5-fluoropyridin-2-amine (500 mg, 4.46 mmol) in tetrahydrofuran (25 ml) at room temperature and the reaction mixture was stirred at 40 °C for 30 min. Then to the reaction mixture was added methyl iodide (696.9 mg, 4.91 mmol) at -40 °C and the resulting mixture was stand at room temperature overnight with stirring. The reaction was quenched by the addition of water and whole mixture was extracted with ethyl acetate. The organic extracts were dried over sodium sulfate and concentrated. The residue was purified by flush column chromatography on silica gel eluting with hexane/ethyl acetate (4/1) to afford 129 mg (23%) of the title compound: 1H-NMR (CDCl3) No. 7.97 (1 H, d, J = 2.6 Hz), 7.28-7.17 (1 H, m), 6.34 (1 H, dd, J = 3.5, 9.1 Hz), 2.90 (3H, d, J = 5.1 Hz).
References:
WO2005/105732,2005,A1 Location in patent:Page/Page column 57-58
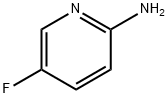
21717-96-4
431 suppliers
$10.00/1g

74-88-4
357 suppliers
$15.00/10g

868636-72-0
47 suppliers
$110.00/100mg