
5-Fluoro-thiazolo[5,4-b]pyridin-2-ylaMine synthesis
- Product Name:5-Fluoro-thiazolo[5,4-b]pyridin-2-ylaMine
- CAS Number:865663-86-1
- Molecular formula:C6H4FN3S
- Molecular Weight:169.18
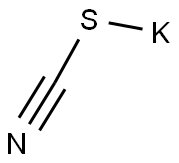
333-20-0
459 suppliers
$13.50/100G
1827-27-6
318 suppliers
$5.00/1g
![5-Fluoro-thiazolo[5,4-b]pyridin-2-ylaMine](/CAS/20150408/GIF/865663-86-1.gif)
865663-86-1
15 suppliers
$90.00/50mg
Yield:865663-86-1 69.2%
Reaction Conditions:
with bromine;acetic acid at 0 - 20; for 16.5 h;
Steps:
4.A
EXAMPLE 4; (3R * 4R *)-N-(5-Fluorothiazolo[5, 4-b]pyridin-2-yl)-4 -1- azaspiro[bicyclo 2.2.1 ]heptane-3, 5 '-οχαζο J -2 '-amine; Step A: 5-Fluorothiazolo[5,4-b]p ridin-2-amine; 6-Fluoropyridin-3 -amine (4 g, 35.7 mmol) was added to a mechanically stirred suspension of potassium rhodanate (27.7 g, 285 mmol) in acetic acid (89 mL) at 0 °C. The flask 3 -neck flask was then fitted with an addition funnel charged with bromine (5.70 mL, 1 1 1 mmol) in acetic acid (29.7 mL). The bromine solution was added over 30 min and the solution turned into a viscous and yellow mixture. After bromine addition was complete, the reaction mixture was allowed to warm to ambient temperature and stirred at that temperature for 16 h. Water (30 mL) was added to the reaction mixture and it was heated to 85 °C for 20 min before the solids were filtered and washed with water and methanol to give 5-fluorothiazolo[5,4- b]pyridin-2-amine (4.18 g, 24.71 mmol, 69.2 % yield) as a yellow solid.
References:
WO2011/56503,2011,A1 Location in patent:Page/Page column 38
6628-77-9
353 suppliers
$8.00/5g

333-20-0
459 suppliers
$13.50/100G
![5-Fluoro-thiazolo[5,4-b]pyridin-2-ylaMine](/CAS/20150408/GIF/865663-86-1.gif)
865663-86-1
15 suppliers
$90.00/50mg