
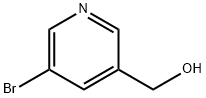
(5-(FURAN-2-YL)PYRIDIN-3-YL)METHANOL synthesis
- Product Name:(5-(FURAN-2-YL)PYRIDIN-3-YL)METHANOL
- CAS Number:887973-98-0
- Molecular formula:C10H9NO2
- Molecular Weight:175.18
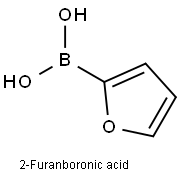
13331-23-2
342 suppliers
$13.43/250mgs:
37669-64-0
213 suppliers
$12.00/1g

887973-98-0
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with caesium carbonate;lithium chloride;dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;palladium diacetate in 1,4-dioxane at 20; for 1.5 h;Heating / reflux;
Steps:
185
To a solution of 0.10 g of (5-bromopyridin-3-yl)methanol in 5 mL of dioxane, 72 mg of 2-furanboronic acid, 67 mg of lithium chloride, 0.51 g of cesium carbonate, 5.4 mg of palladium acetate and 20 mg of 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl were added at room temperature, and the mixture was heated under reflux for 1 hour 30 minutes under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, and the solvent was then distilled off under reduced pressure. The resultant residue was purified by flash silica gel column chromatography using gradient elution with chloroform:methanol = 95:5 to 90:10 to obtain 39 mg of (5-(2-furyl)pyridin-3-yl)methanol as a yellow oily substance. 1H-NMR (CDCl3) δ: 1.50-1.80 (1H, broad), 4.78 (2H, s), 6.52 (1H, dd, J = 3.4, 1.7 Hz), 6. 77 (1H, d, J = 3.4 Hz), 7.53 (1H, d, J = 1.7 Hz), 7.98 (1H, s), 8.47 (1H, d, J = 1.8 Hz), 8.85 (1H, d, J = 1.8 Hz)
References:
EP2022793,2009,A1 Location in patent:Page/Page column 96