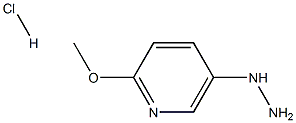
5-Hydrazinyl-2-Methoxypyridine hydrochloride synthesis
- Product Name:5-Hydrazinyl-2-Methoxypyridine hydrochloride
- CAS Number:179543-88-5
- Molecular formula:C6H10ClN3O
- Molecular Weight:175.6161
6628-77-9

179543-88-5
The general procedure for the synthesis of 5-hydrazino-2-methoxypyridine hydrochloride from 5-amino-2-methoxypyridine is as follows: 1. Dissolve sodium nitrite (3.795 g) in water (20 ml) to prepare a solution. 2. the above solution was added slowly dropwise to a concentrated hydrochloric acid solution of 5-amino-2-methoxypyridine (6.21 g) while cooling in an ice bath. 3. Hydrochloric acid (50 ml) was added slowly over a period of 60 minutes and the reaction mixture was continued to be stirred at the same temperature for 30 minutes. 4. A concentrated hydrochloric acid solution of tin(II) chloride dihydrate (39.5 g) was prepared. 5. Hydrochloric acid (30 ml) was added dropwise over a period of 30 minutes at an internal temperature of about 10°C in the reaction solution, followed by stirring of the reaction mixture for 2 hours at room temperature. 6. Phase separation was carried out by adding aqueous (300 ml) solution of sodium hydroxide (75 g) and diethyl ether to the reaction solution under cooling in an ice bath. 7. The aqueous phase was extracted twice with diethyl ether, after which the aqueous phase was saturated with sodium chloride and again extracted with diethyl ether. 8. All organic phases were combined and dried with anhydrous sodium sulfate. 9. After filtration, an ethanolic solution of 1 M hydrochloric acid (50 ml) was added to the filtrate and the mixture was stirred. 10. The solid precipitate formed was collected by filtration, washed with diethyl ether and dried to give the target product 5-hydrazino-2-methoxypyridine hydrochloride (5.02 g, 57% yield). Product characterization data: 1H-NMR (400 MHz, DMSO-d6) δ: 3.81 (3H, s), 6.82 (1H, d, J = 8.8 Hz), 7.57 (1H, dd, J = 8.8, 2.9 Hz), 7.97 (1H, d, J = 2.9 Hz), 8.55-9.20 (1H, br), 10.13-10.50 (3H, br). MS (ESI) m/z: 140 (M + H)+.
160664-95-9
60 suppliers
inquiry

179543-88-5
53 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in ethanol
Steps:
1
[Referential Example 1] ;5-Hydrazino-2-methoxypyridine hydrochloride; [] A solution of sodium nitrite (3.795 g) in water (20 mL) was added dropwise to 5-amino-2-methoxypyridine (6.21 g) in concentrated hydrochloric acid (50 mL) over a period of 60 minutes with ice cooling, and the resultant mixture was stirred at a constant temperature for 30 minutes. Tin(II) chloride dihydrate (39.5 g) in concentrated hydrochloric acid (30 mL) was added dropwise to the reaction mixture at an internal temperature of about 10°C for 30 minutes, followed by stirring for 2 hours at room temperature. Under cooling with ice, the reaction mixture was partitioned between sodium hydroxide (75 g) in water (300 mL) and diethyl ether. The aqueous layer was extracted with diethyl ether twice. Subsequently, the aqueous layer was saturated with sodium chloride, followed by extraction with diethyl ether. The organic layers were combined, and dried over sodium sulfate anhydrate, followed by filtration. 1M HCl in ethanol (50 mL) was added to the filtrate and the mixture was stirred. The solid that precipitated was collected by filtration, washed with diethyl ether, and dried, to thereby give the title compound (5.02 g, 57%).1H-NMR(400MHz,DMSO-d6)δ: 3.81(3H,s), 6.82(1H,d,J=8.8Hz), 7.57 (1H, dd, J=8.8,2.9Hz) , 7.97 (1H, d, J=2.9Hz) , 8.55-9.20 (1H, br) , 10.13-10.50(3H,br). MS(ESI)m/z: 140(M+H)+.
References:
DAIICHI PHARMACEUTICAL CO., LTD. EP1591443, 2005, A1 Location in patent:Page/Page column 24

6628-77-9
357 suppliers
$8.00/5g

179543-88-5
53 suppliers
inquiry