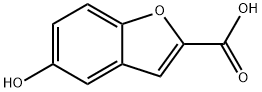
5-HYDROXY-1-BENZOFURAN-2-CARBOXYLIC ACID synthesis
- Product Name:5-HYDROXY-1-BENZOFURAN-2-CARBOXYLIC ACID
- CAS Number:56172-36-2
- Molecular formula:C9H6O4
- Molecular Weight:178.14
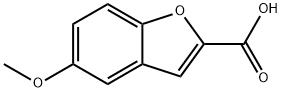
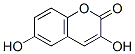
10242-08-7

56172-36-2
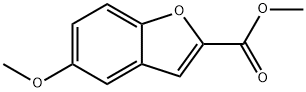
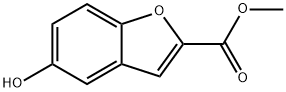
Step 1: Preparation of 5-hydroxybenzofuran-2-carboxylic acid. 5-Methoxybenzofuran-2-carboxylic acid (1.0 g, 5.2 mmol) was dissolved in anhydrous dichloromethane (25 ml) and the solution was cooled to -78°C. A 1 M solution of boron tribromide dichloromethane (15.6 ml) was added slowly and dropwise under nitrogen protection. The reaction mixture was gradually warmed to room temperature and stirred continuously at this temperature for 4 hours. Upon completion of the reaction, the reaction was quenched with aqueous ammonium chloride solution (20 ml) and subsequently extracted with ethyl acetate. The organic layer was washed with water and dried over anhydrous sodium sulfate. After treatment, 0.9 g (100% yield) of the crude product of 5-hydroxybenzofuran-2-carboxylic acid was obtained.1H NMR (CD3OD) δ = 6.84 (dd, 1H), 6.95 (d, 1H), 7.17 (s, 1H), 7.32 (d, 1H).

10242-08-7
123 suppliers
$18.00/250mg

56172-36-2
35 suppliers
$45.00/100mg
Yield:56172-36-2 100%
Reaction Conditions:
with boron tribromide in dichloromethane at -78 - 20; for 4 h;
Steps:
1.1 Preparation of 4.6-dibromo-2-carboxy-5-hydroxybenzofuran
Step 1: Preparation of 2-carboxy-5-hydroxybenzofuran. 2-Carboxy-5-methoxybenzofuran (1.0 g, 5.2 mmol) was dissolved in anhydrous dichloromethane (25 ml) and cooled to -78° C. A 1 M solution of borontribromide (15.6 ml) in dichloromethane was added slowly. The reaction mixture was allowed to warm to ambient temperature under an atmosphere of nitrogen, and stirred 4 hours. The solution was quenched with aqueous ammonium chloride (20 ml) and extracted with ethyl acetate. The aqueous layer was washed with water and dried over sodium sulfate. 0.9 g (100%) of crude product was obtained. 1H NMR(CD3OD) δ=6.84 (dd, 1H), 6.95 (d, 1H), 7.17 (s, 1H), 7.32 (d, 1H).
References:
US2005/26969,2005,A1 Location in patent:Page/Page column 12
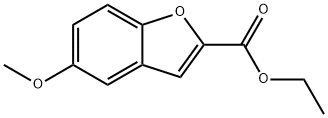
50551-56-9
63 suppliers
$26.00/250mg

56172-36-2
35 suppliers
$45.00/100mg
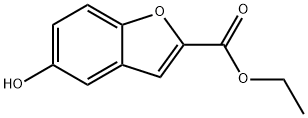
99370-68-0
16 suppliers
inquiry

56172-36-2
35 suppliers
$45.00/100mg