
5-hydroxymethyl-2-phenyl-7-nitro-1H-indole synthesis
- Product Name:5-hydroxymethyl-2-phenyl-7-nitro-1H-indole
- CAS Number:1120334-32-8
- Molecular formula:C15H12N2O3
- Molecular Weight:268.27

1120334-31-7
10 suppliers
inquiry

1120334-32-8
1 suppliers
inquiry
Yield:1120334-32-8 71 %
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran at 0 - 20;
Steps:
1 Preparation Example 1: (7-nitro-2-phenyl-1H-indol-5-yl) methanol {(7-nitro-2-phenyl-1H-indol-5-yl) methanol}
7-Nitro-2-phenyl-1H-indole-5-carboxylic acid(1.0 g, 3.45 mmol) was added to THF (10 mL) andcooled to 0° C.After slowly adding 2M BH3·SMe2in THF (5.3 mL, 10.6 mmol) dropwise, the mixture was stirred at the same temperature for 30 minutes, heated to room temperature, and stirred for another 5 hours.The reaction solution was cooled to 0°C and 1N NaOH aqueous solution (10 mL) was slowly added dropwise over 30 minutes.After stirring for an additional hour and extracting twice by adding EA, the organic layer was collected and washed with a saturated NaCl aqueous solution.The organic layer was dried over MgSO4and filtered.The filtrate was concentrated under reduced pressure, crystallized by adding EtOH to the concentrated residue, filtered, and vacuum dried toobtain the title compound (770 mg, 71%) as a yellow solid.
References:
WO2023/13996,2023,A1 Location in patent:Paragraph 194-197
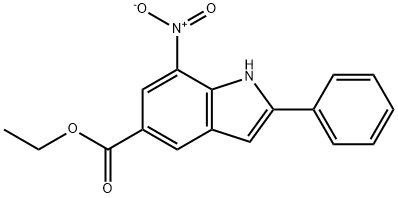
1120334-30-6
13 suppliers
inquiry

1120334-32-8
1 suppliers
inquiry