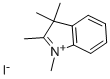
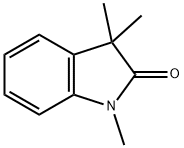
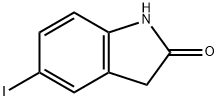
5-Iodo-1,3,3-trimethyl-2-oxoindoline synthesis
- Product Name:5-Iodo-1,3,3-trimethyl-2-oxoindoline
- CAS Number:139487-11-9
- Molecular formula:C11H12INO
- Molecular Weight:301.12
193354-13-1
75 suppliers
$29.00/100mg

74-88-4
352 suppliers
$15.00/10g

139487-11-9
15 suppliers
inquiry
Yield:139487-11-9 74%
Reaction Conditions:
with potassium hydroxide in dimethyl sulfoxide at 20; for 0.5 h;
Steps:
4.2. General procedure for the N-methylation reaction13 of indolin-2-ones 7a-c
5-Iodo-indolin-2-one 7a (50 mg, 193 μmol) and methyl iodide (72.1 μL, 1.16 mmol, 6 equiv) were added to a vigorously stirred solution of KOH (45.1 mg, 0.80 mmol, 4 equiv) in DMSO (2 mL) at room temperature. The reaction was monitored by TLC until complete consumption of 7a (30 min). After water addition, the reaction mixture was extracted with ethyl acetate and the organic layer dried over Na2SO4. Solvent was then evaporated under reduced pressure and the 5-iodo-1,3,3-trimethylindolin-2-one 8a was purified using preparative thin layer chromatography [light petroleum/ethyl acetate (3:2)]. Crystallization from dichloromethane/light petroleum afforded 8a in 74% yield (43 mg).
References:
Menezes, José C.J.M.D.S.;Pereira, Ana M.V.M.;Neves, Maria G.P.M.S.;Silva, Artur M.S.;Santos, Sérgio M.;Martinez, Sabrina T.;Silva, Bárbara V.;Pinto, ?ngelo C.;Cavaleiro, José A.S. [Tetrahedron,2012,vol. 68,# 39,p. 8330 - 8339] Location in patent:experimental part