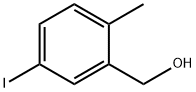
(5-iodo-2-Methylphenyl)Methanol synthesis
- Product Name:(5-iodo-2-Methylphenyl)Methanol
- CAS Number:1260242-01-0
- Molecular formula:C8H9IO
- Molecular Weight:248.06
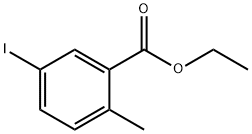
612833-45-1
29 suppliers
$227.46/1g

1260242-01-0
46 suppliers
inquiry
Yield:1260242-01-0 97%
Reaction Conditions:
Stage #1: ethyl 5-iodo-2-methylbenzoatewith lithium borohydride in tetrahydrofuran;Reflux;Inert atmosphere;
Stage #2: with water;ammonium chloride in tetrahydrofuran at 0;
Steps:
3.3
Step 3: (5-Iodo-2-methylphenyl)methanolTo a solution of ester 4 (41.0 g, 141 mmol) in THF (300 mL) was added lithium borohydride (2.0 M in THF, 212 mL). The reaction mixture was refluxed overnight. After cooling to 0 °C, the reaction was quenched by addition of aq. saturated NH4C1 solution. The mixture was diluted with water and extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous MgS04, filtered and evaporated in vacuo to yield the titled compound (34.0 g, 97%) as a white solid, which was carried on to the next step without further purification.[M-OH"]+ 231.
References:
WO2011/159067,2011,A2 Location in patent:Page/Page column 51
27701-23-1
0 suppliers
inquiry

1260242-01-0
46 suppliers
inquiry
79669-49-1
438 suppliers
$5.00/1g

1260242-01-0
46 suppliers
inquiry
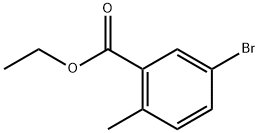
359629-91-7
35 suppliers
$50.00/1g

1260242-01-0
46 suppliers
inquiry