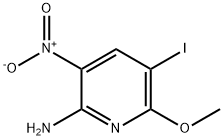
5-Iodo-6-Methoxy-3-nitro-pyridin-2-ylaMine synthesis
- Product Name:5-Iodo-6-Methoxy-3-nitro-pyridin-2-ylaMine
- CAS Number:868539-54-2
- Molecular formula:C6H6IN3O3
- Molecular Weight:295.03
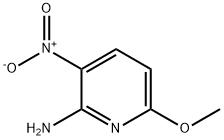
73896-36-3
234 suppliers
$9.00/10g

868539-54-2
9 suppliers
$195.00/1g
Yield:868539-54-2 153 g
Reaction Conditions:
with N-iodo-succinimide;acetic acid at 20 - 27; for 18 h;
Steps:
1 Intermediate 70 5) MeS02Na, Cul, 2-picolinic acid Intermediate 71
Step 1) NIS (199 g, 0.88 mol) was added to a suspension of 6-methoxy-3- nitropyridin-2-amine (Intermediate 70, 100 g, 0.59 mol) in acetic acid (1.9 L), the reaction mixture was stirred at rt for 18 h, concentrated in vacuo and partitioned between aqueous sodium hydroxide (1M, 2 L) and EtOAc (2.5 L). The phases were separated and the organic layer was washed with saturated aqueous NaHC03 (1.5 L), and brine (1 L), dried (Na2S04), and concentrated in vacuo. The residue was triturated with heptane (1.5 L) and dried in vacuo to yield 5-iodo-6-methoxy-3-nitropyridin-2- amine (153 g, 0.52 mol) as a yellow solid. (0447) LCMS (Method 8): m/z 293.9 (ES-), at 2.11 min. (0448) 1H NMR: (300 MHz, DMSO-i) δ: ppm 3.89 (s, 3H), 8.19 (br s, 2H), 8.52 (s, 1H).
References:
WO2019/86902,2019,A1 Location in patent:Page/Page column 50
38533-61-8
311 suppliers
$8.00/5g

868539-54-2
9 suppliers
$195.00/1g