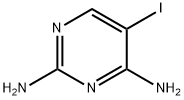
5-Iodo-pyrimidine-2,4-diamine synthesis
- Product Name:5-Iodo-pyrimidine-2,4-diamine
- CAS Number:157924-46-4
- Molecular formula:C4H5IN4
- Molecular Weight:236.01
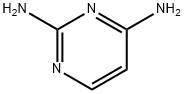
156-81-0

157924-46-4
General procedure for the synthesis of 5-iodopyrimidine-2,4-diamine from 2,4-diaminopyrimidine: To a flame-dried 100 mL round-bottomed flask was added a methanol (MeOH, 30 mL) solution of 2,4-diaminopyrimidine (1.0 g, 9.08 mmol), followed by the slow dropwise addition of iodine chloride (ICl, 30 mL, 29.06 mmol). The reaction mixture was stirred at 25 °C for 15 h. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The resulting viscous oily substance was stirred with ether (Et2O, 40 mL) for 45 min. The resulting solid was collected by filtration and washed with ether (3 × 10 mL) to give the hydrochloride salt (3.14 g) as a yellow solid. The crude salt was suspended in a 1.0 N sodium hydroxide (NaOH, 100 mL) solution and stirred at 25 °C for 2 h. The solid product was collected by filtration and was washed with ether (3 × 10 mL). The solid product was filtered, washed with water (2 x 10 mL) and dried to give 2,4-diamino-5-iodopyrimidine (1.71 g, 80% yield) in brown powder form. For the preparation of analytical samples, the product was recrystallized from acetonitrile (MeCN) to give 2,4-diamino-5-iodopyrimidine in the form of colorless crystals: thin-layer chromatography (TLC) Rf = 0.25 (unfolding reagent in the ratio of chloroform:methanol 9:1); the melting point is 212-214 °C. Nuclear magnetic resonance hydrogen spectrum (1H NMR, DMSO-d6) δ 7.92 (s, 1H), 6.40 (s, 2H), 6.10 (s, 2H); nuclear magnetic resonance carbon spectrum (13C NMR, DMSO-d6) δ 162.8, 162.7, 162.0, 61.2; high-resolution electron bombardment mass spectrometry (HREI) [M+] 235.9559 ( Calculated value C4H5IN4: 235.9559); Elemental analysis (C4H5IN4) C, H, N content in accordance with theoretical values.

156-81-0
218 suppliers
$19.00/1g

157924-46-4
31 suppliers
inquiry
Yield:157924-46-4 97%
Reaction Conditions:
Stage #1: pyrimidine-2,4-diaminewith periodic acid;sulfuric acid;iodine;acetic acid in water monomer; for 5.5 h;
Stage #2: with potassium hydroxide in water monomer at 20;
References:
Wellington, Kevin W.;Ooi, Hua Chee;Benner, Steven A. [Nucleosides, nucleotides and nucleic acids,2009,vol. 28,# 4,p. 275 - 291] Location in patent:experimental part