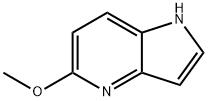
5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE synthesis
- Product Name:5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE
- CAS Number:17288-40-3
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
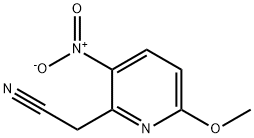
111795-99-4
![5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/17288-40-3.gif)
17288-40-3
GENERAL METHOD: 6-methoxy-3-nitropyridine-2-acetonitrile derivative 13a or 13b (12.9 mmol) was dissolved in ethanol or methanol (40 mL) and a catalytic amount of 10% palladium carbon was added. The reaction mixture was placed in a Paar hydrogenation unit and hydrogenated at room temperature for 24 hours. Upon completion of the reaction, the catalyst was removed by filtration and the solvent was removed by concentration under reduced pressure. The resulting residue was purified by column chromatography with the eluent dichloromethane/ethyl acetate (95/5 for compound 14a) or (6/4 for compound 14b).
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/17288-40-3.gif)
17288-40-3
141 suppliers
$22.00/250mg
Yield: 95%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol;ethyl acetate under 3102.97 Torr; for 6 h;Parr apparatus;
Steps:
17.1
A suspension of (5-methoxy-3-nitro-pyridin-2-yl)-acetonitrile (Maybridge, 986 mg, 5.1 mmol) and Pd/C (10%, 986 mg) in EtOH/EtOAc (95/5, 50 mL) was shaken for 6 hours under H2 (60 PSI) in Parr apparatus. The reaction mixture was then filtered through a celite pad, and the filter cake was washed with EtOAc (20 mL). The filtrate was concentrated, and the residue was dissolved in EtOAc (50 mL), washed with NaHCO3 (saturated solution, 50 mL), dried over MgSO4, filtered, concentrated, and purified via flash chromatography (hexane/EtOAc), affording 5-Methoxy-1H-pyrrolo[3,2-b]pyridine as a white solid (720 mg, 95% yield).
References:
Roche Palo Alto LLC US2007/123535, 2007, A1 Location in patent:Page/Page column 42

111795-99-4
30 suppliers
$45.00/1mg
![5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/17288-40-3.gif)
17288-40-3
141 suppliers
$22.00/250mg

570386-39-9
0 suppliers
inquiry
![5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/17288-40-3.gif)
17288-40-3
141 suppliers
$22.00/250mg
3598-13-8
129 suppliers
$17.00/10g
![5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/17288-40-3.gif)
17288-40-3
141 suppliers
$22.00/250mg
5446-92-4
311 suppliers
$10.00/5g
![5-METHOXY-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/17288-40-3.gif)
17288-40-3
141 suppliers
$22.00/250mg