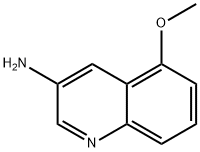
5-methoxyquinolin-3-amine synthesis
- Product Name:5-methoxyquinolin-3-amine
- CAS Number:881668-93-5
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
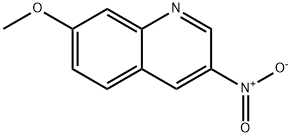
39977-95-2
5 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

881668-93-5
20 suppliers
$65.00/10mg
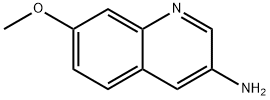
87199-83-5
42 suppliers
$110.00/50mg
Yield: 12%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethyl acetate at 20;
Steps:
20
0333] A mixture of 5- and 7-methoxy substituted 3-nitroquinolines (780 mg,3.82 mmol) was dissolved in EtOAc (75 mL) and the reaction mixture sparged with N2 for several minutes. 10% Pd/C (54 mg) was then added and a H2 balloon was connected to the reaction flask. The reaction mixture was sparged with H2 and stirred at room
References:
NOVARTIS AG WO2007/84786, 2007, A1 Location in patent:Page/Page column 117-118