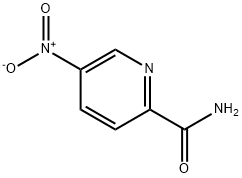
5-Nitropyridine-2-carboxamide synthesis
- Product Name:5-Nitropyridine-2-carboxamide
- CAS Number:59290-34-5
- Molecular formula:C6H5N3O3
- Molecular Weight:167.12
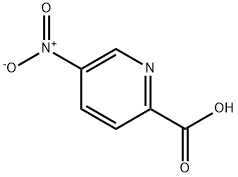
30651-24-2
229 suppliers
$14.14/1gm:

59290-34-5
31 suppliers
$175.00/2.5g
Yield:-
Reaction Conditions:
with pyridine;ammonium chloride;2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide in ethyl acetate
Steps:
1.1A [0480] General Method 5B: Amide Coupling using T3P/ Pyridine
General procedure: A solution of the appropriate carboxylic acidcarboxylic acid hydrochloride (1 eq.) and the appropriate amine or amine hydrochloride (1.1-1.9 eq.) in pyridine (about 0.1 M) was heated to 60° C., and T3P (50% in ethyl acetate, 1.5-15 eq.) was added dropwise. Alternatively, T3P was added at RT and the mixture was then stirred at RTheated to 50 to 90° C. After 1 to 20 h, the reaction mixture was cooled to RT and either purified directly by meanspreparative RP-HPLC (water-acetonitrile gradient or water- methanol gradient) or admixed with water and ethyl acetate. The aqueous phase was extracted with ethyl acetate.combined organic phases were washed with aqueous buffer solution (pH=5), with saturated aqueous sodium hydrogen- carbonate solution and with saturated aqueous sodium chloride solution, dried over sodium sulphate and concentrated under reduced pressure. The crude product was then optionally purified either by means of normal phase chromatography (eluent: cyclohexane/ethyl acetate mixtures or dichloromethane/methanol mixtures) or preparative RP-HPLC(water/acetonitrile gradient or water/methanol gradient); According to General Method 58, 4.00 g (23.8 mmol) of 5-nitropyridine-2-carboxylic acid and 1.91 g (35.7 mmol, 1 .5 eq.) of ammonium chloride were reacted. After workup, the crude product was used for the next stage without thrther purification. LC/MS [Method 1]: R=0.39 mm; MS (ESIpos):mlz=168 (M+H),
References:
BAYER PHARMA AKTIENGESELLSCHAFT;RÖHRIG, Susanne;JIMENEZ-NUNEZ, Eloisa;SCHLEMMER, Karl-Heinz;TERSTEEGEN, Adrian;TELLER, Henrik;HILLISCH, Alexander;HEITMEIER, Stefan;SCHMIDT, Martina Victoria;ACKERSTAFF, Jens;STAMPFUß, Jan US2017/291892, 2017, A1 Location in patent:Paragraph 0480; 0481; 0498; 0499; 0500
4214-76-0
523 suppliers
$6.00/10g

59290-34-5
31 suppliers
$175.00/2.5g