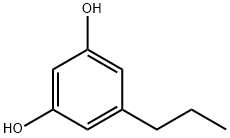
5-propylbenzene-1,3-diol synthesis
- Product Name:5-propylbenzene-1,3-diol
- CAS Number:500-49-2
- Molecular formula:C9H12O2
- Molecular Weight:152.19
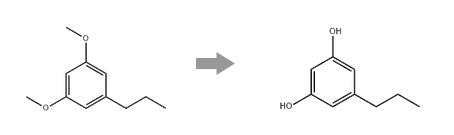
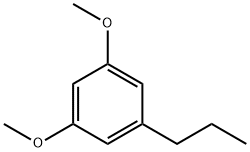
41395-10-2
14 suppliers
inquiry

500-49-2
97 suppliers
$80.00/500mg
Yield:500-49-2 99%
Reaction Conditions:
with hydrogen bromide;acetic acid in water at 125; for 3 h;Inert atmosphere;
Steps:
5-propylbenzene-1,3-diol (19a).
In a 100 mL round bottom flask, open to the atmosphere was added the crude product SI-2 (0.736 g, 4.08 mmol, 1.0 equiv.) and a 1:1 mixture of glacial acetic acid (20 mL, 349.7 mmol, 17.4 M) and hydrobromic acid (48% in H2O, 20 mL, 368.3 mmol, 18.4 M). The reaction mixture was refluxed at 125 °C for 3 hours while stirring, or until the starting material was consumed via TLC analysis. At this point, the reaction was allowed to cool to room temperature and quenched by the addition of DI H2O. The biphasic solution was added to a separatory funnel, wherein the organic portion was extracted Et2O (ca. 3x 20 mL). The organic layers were then combined, neutralized with a concentrated sodium bicarbonate solution (ca. 30 mL), washed with a saturated brine solution (ca. 50 mL), dried over MgSO4, filtered, and concentrated in vacuo to afford the final product without purification (0.609 g, 99% yield) as a pale yellow oil.
References:
Golliher, Alexandra E.;Tenorio, Antonio J.;Dimauro, Nina O.;Mairata, Nicolas R.;Holguin, F. Omar;Maio, William [Tetrahedron Letters,2021,vol. 67,art. no. 152891] Location in patent:supporting information
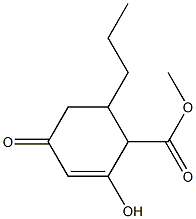
27871-91-6
8 suppliers
inquiry

500-49-2
97 suppliers
$80.00/500mg
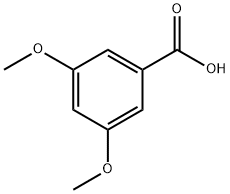
1132-21-4
374 suppliers
$10.00/5g

500-49-2
97 suppliers
$80.00/500mg
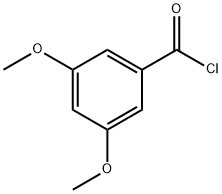
17213-57-9
130 suppliers
$7.00/1g

500-49-2
97 suppliers
$80.00/500mg
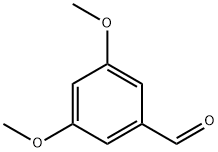
7311-34-4
329 suppliers
$10.00/1g

500-49-2
97 suppliers
$80.00/500mg