
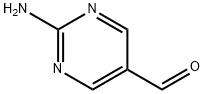
5-Pyrimidinecarboxaldehyde, 2-amino-, oxime (9CI) synthesis
- Product Name:5-Pyrimidinecarboxaldehyde, 2-amino-, oxime (9CI)
- CAS Number:862096-02-4
- Molecular formula:C5H6N4O
- Molecular Weight:138.13
120747-84-4
186 suppliers
$11.00/100mg

862096-02-4
8 suppliers
inquiry
Yield:862096-02-4 2.1 g
Reaction Conditions:
with pyridine;hydroxylamine hydrochloride in ethanol at 20; for 16 h;
Steps:
Preparation of 5-iodo-3-(2-aminopyrimidin-5-yl)isoxazole 14b
A suspension of 2-aminopyrimidine-5-carboxyaldehyde 18 (1.60 g, 13.0 mmol), hydroxylamine hydrochloride (0.99 g, 14.3 mmol) and pyridine (1.15 mL, 14.3 mmol) in ethanol (50 mL) was stirred at room temperature for 16 hours. The reaction mixture was quenched by adding saturated NaHCO3 (15 mL) and filtered the precipitate. The precipitate was washed with distilled water to yield compound 16b (2.10 g). N-Chlorosuccimide (2.03g, 15.2 mmol) was added to a suspension of 16b (2.1 g, 15.2 mmol), ethynyl tri-n-butylstannane (4.31 g, 13.7 mmol), NaHCO3 (3.19 g, 38 mmol), ethyacetate (100 mL) and distilled water (5 mL). After stirring at room temperature for 2 hours, distilled water (50 mL) was added to the reaction mixture. After the removal of the insoluble particles using filtration, the organic layer was washed with saturated NaHCO3 (50 mL) and brine (50 mL) and dried over MgSO4. The solvent was removed in vacuo and the residue was used in the next reaction without further purification. The crude 17b was dissolved in THF (30 mL). Iodine (5.0 g, 19.7 mmol) was added to the solution at room temperature. After stirring at room temperature for 45 minutes, the reaction mixture was diluted with 6% sodium thiosulfate solution (100 mL) and extracted with ethylacetate (100 ml). The organic layer was washed with brine and dried over MgSO4. The solvent was removed in vacuo and the residue was recrystallized from n-hexane to yield compound 14b (0.74 g, 17% for two steps) as a pale brown powder
References:
Sugimoto, Tomohiro;Shimazaki, Yoichi;Manaka, Akira;Tanikawa, Tetsuya;Suzuki, Keiko;Nanaumi, Kayoko;Kaneda, Yoshie;Yamasaki, Yukiko;Sugiyama, Hiroyuki [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 17,p. 5739 - 5743] Location in patent:supporting information; experimental part