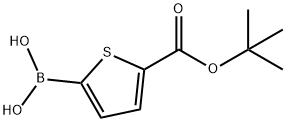
5-TERT-BUTOXYCARBONYLTHIOPHENE-2-BORONIC ACID synthesis
- Product Name:5-TERT-BUTOXYCARBONYLTHIOPHENE-2-BORONIC ACID
- CAS Number:925921-29-5
- Molecular formula:C9H13BO4S
- Molecular Weight:228.07
62224-20-8

5419-55-6

925921-29-5
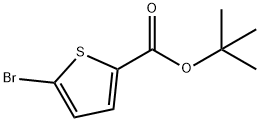
General procedure for the synthesis of (5-(tert-butoxycarbonyl)thiophen-2-yl)boronic acid from tert-butyl 5-bromothiophene-2-carboxylate and triisopropyl borate: tert-butyl 5-bromothiophene-2-carboxylate (19 g, 72 mmol) was added drop-wise to tetrahydrofuran (THF, 400 mL) solution of tert-butyl 5-bromothiophene-2-carboxylate (29 mL, 72 mmol) at -78 °C. After dropwise addition, the reaction mixture was continued to be stirred for 30 min, followed by slow addition of triisopropyl borate (15 g, 79 mmol). After 2 hours of reaction, the reaction was quenched by the addition of saturated aqueous ammonium chloride (NH4Cl, 100 mL) and the mixture was gradually warmed to room temperature. Upon completion of the reaction, the organic and aqueous layers were separated, and the organic layer was dried over anhydrous sodium sulfate (Na2SO4), concentrated and purified by silica gel column chromatography (eluent: 0-75% ethyl acetate/hexanes) to afford the target product, (5-(tert-butoxycarbonyl)thiophen-2-yl)boronic acid (8.9 g, 56% yield), as a brown solid. Its 1H NMR (500 MHz, DMSO-d6) data were as follows: δ 7.71 (d, J=3.5 Hz, 1H), 7.49 (d, J=3.5 Hz, 1H), 1.49 (s, 9H).

62224-20-8
19 suppliers
$375.00/1g

5419-55-6
388 suppliers
$12.00/5g

925921-29-5
20 suppliers
$45.00/50mg
Yield:925921-29-5 56%
Reaction Conditions:
Stage #1: 2-bromothiophene-5-carboxylic acid tert-butyl esterwith n-butyllithium in tetrahydrofuran at -78; for 0.5 h;
Stage #2: Triisopropyl borate in tetrahydrofuran; for 2 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran at 20;
Steps:
4
Synthesis of 3; To 2 (19 g, 72 mmol) in THF (400 mL) at -78 0C was added n-butyllithium (29 mL, 72 mmol) dropwise. Upon complete addition the reaction was stirred for 30 min and triisopropyl borate (15 g, 79 mmol) was added dropwise. After 2 h was quenched by the addition of satd. aq. NH4CI (100 mL) and warmed to rt. The layers were separated and the organics were dried over Na2SO4, concentrated and purified by chromatography (silica gel, 0-75% ethyl acetate/hexanes) to obtain 3 (8.9 g, 56%) as a brown solid: 1H NMR (500 MHz, DMSO-d6) δ 7.71 (d, J = 3.5 Hz, 1 H), 7.49 (d, J = 3.5 Hz, 1 H), 1.49 (s, 9H).
References:
WO2007/18941,2007,A2 Location in patent:Page/Page column 116-117