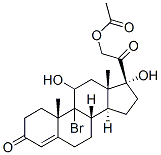
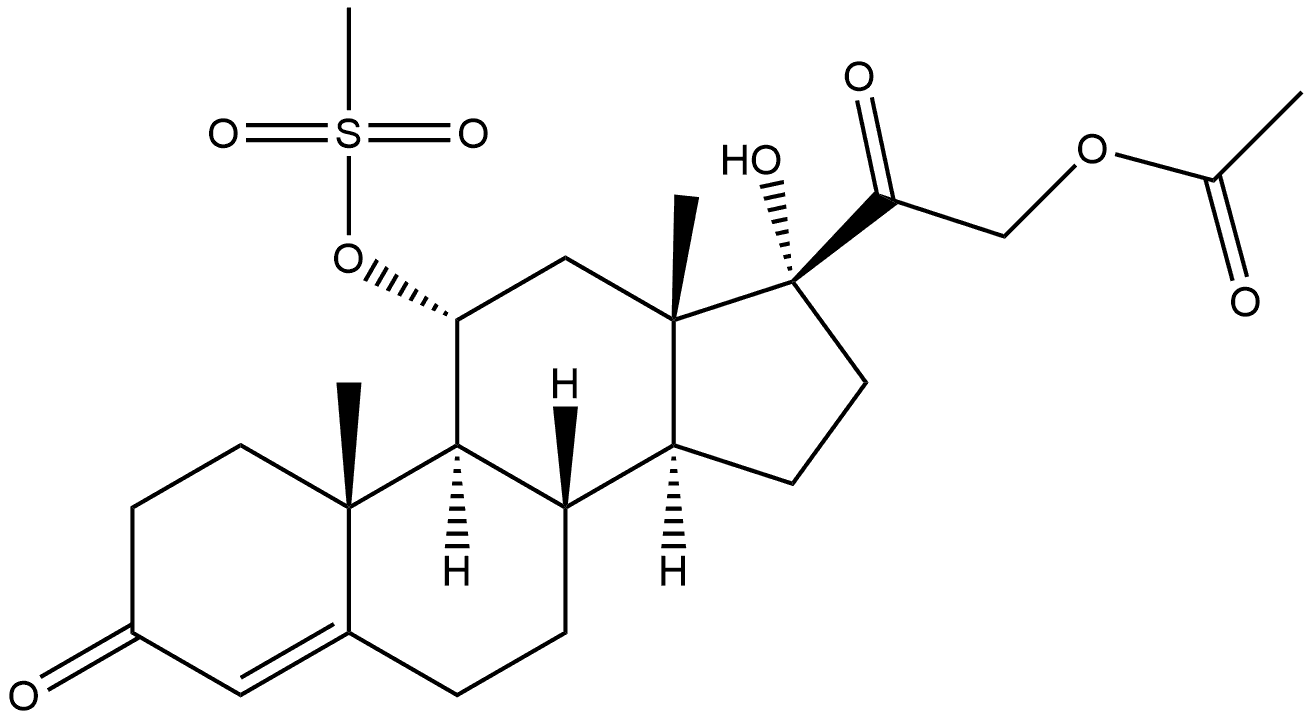
9-Bromo-11,17,21-trihydroxypregn-4-ene-3,20-dione 21-acetate synthesis
- Product Name:9-Bromo-11,17,21-trihydroxypregn-4-ene-3,20-dione 21-acetate
- CAS Number:50733-54-5
- Molecular formula:C23H31BrO6
- Molecular Weight:483.39
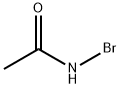
7753-60-8
148 suppliers
$60.00/25g

50733-54-5
25 suppliers
inquiry
Yield:50733-54-5 67 g
Reaction Conditions:
with tetrafluoroboric acid;1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;water in acetone at -5;Reagent/catalyst;Temperature;
Steps:
1.1-1.3 1) Bromo-hydroxyl reaction
50 grams of 17α-hydroxypregna-4,9(11)-diene-3,20-dione-21-acetate (1) is added to 1500 ml of acetone, and the mass concentration is 10%50ml fluoroboric acid aqueous solution, cool to -5, add 60g of dibromodimethylhydantoin and stir to react. After the reaction is completed, neutralize with 10% sodium carbonate aqueous solution by mass concentration, concentrate the organic solvent, and hydrate. , Filtered to obtain 67 g of intermediate (2).
References:
CN111518151,2020,A Location in patent:Paragraph 0026-0027; 030-0031; 0034-0035
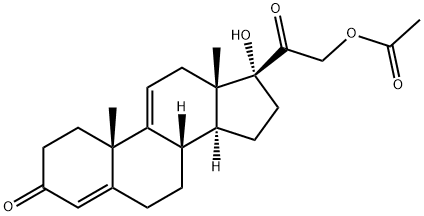
566-35-8
59 suppliers
$495.52/5MG

50733-54-5
25 suppliers
inquiry
113862-93-4
0 suppliers
inquiry

50733-54-5
25 suppliers
inquiry