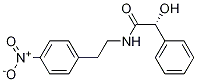
(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide synthesis
- Product Name:(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide
- CAS Number:521284-19-5
- Molecular formula:C16H16N2O4
- Molecular Weight:300.31
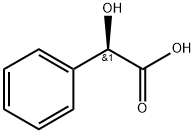
611-71-2
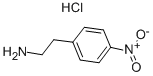
29968-78-3
![(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide](/CAS/GIF/521284-19-5.gif)
521284-19-5
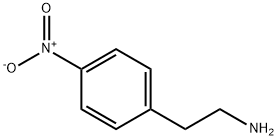
GENERAL STEPS: To a stirred solution of (R)-mandelic acid (97.61 g) in acetonitrile (1000 mL) was slowly added trimethyl borate (66.66 g) at 28 °C (±2 °C). After addition, the reaction system was warmed to 58 °C (±2 °C) and maintained for 90 min. Maintaining this temperature, 4-nitrophenylethylamine hydrochloride (100.0 g) was added to the reaction solution. Subsequently, N,N-diisopropylethylamine (82.92 g) was added slowly and the reaction mixture was heated to reflux for 12 hours. After completion of the reaction, the system was cooled to 30 °C (±2 °C) and diluted with ethyl acetate (1100 mL) and 1 M aqueous hydrochloric acid (1400 mL). The organic phase was separated and washed with 1M aqueous hydrochloric acid solution, 5% aqueous sodium hydroxide solution and saturated saline in turn. The organic phase was concentrated under reduced pressure and the residue was recrystallized from toluene (500 mL). The resulting solid was washed with toluene and dried under vacuum at 48 °C (±2 °C) to give the light yellow crystalline product (R)-2-hydroxy-N-[2-(4-nitrophenyl)ethyl]-2-phenylacetamide. Yield: 125 g (84.3%); HPLC purity: 98.65%.

611-71-2
640 suppliers
$13.00/5g

29968-78-3
445 suppliers
$18.00/5g
![(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide](/CAS/GIF/521284-19-5.gif)
521284-19-5
161 suppliers
$7.00/250mg
Yield:521284-19-5 93.7%
Reaction Conditions:
with benzotriazol-1-ol;N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
1 Example 1: Preparation of compound of formula (V)
200 g of the compound of formula (VII) and 286 g of compound of formula (VI) were added to 1.6 kg of N,N-dimethylformamide, and 132 g of triethylamine, 178 g of 1-hydroxybenzotriazole and 264 g of EDCI were added at room temperature. The reaction was stirred at room temperature for 1 h, 1.6 kg of purified water was added, stirred for 2 h for crystallization, filtered and dried to obtain 371 g of the compound of formula (V) with a yield of 93.7%.
References:
CN113880720,2022,A Location in patent:Paragraph 0038-0040
24954-67-4
82 suppliers
inquiry
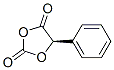
54256-33-6
4 suppliers
inquiry
![(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide](/CAS/GIF/521284-19-5.gif)
521284-19-5
161 suppliers
$7.00/250mg

24954-67-4
82 suppliers
inquiry

611-71-2
640 suppliers
$13.00/5g
![(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide](/CAS/GIF/521284-19-5.gif)
521284-19-5
161 suppliers
$7.00/250mg

611-71-2
640 suppliers
$13.00/5g
![(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide](/CAS/GIF/521284-19-5.gif)
521284-19-5
161 suppliers
$7.00/250mg