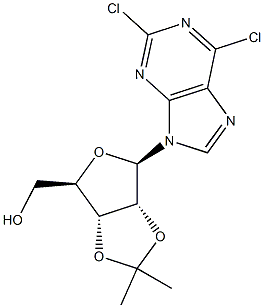
2,6-Dichloro-9-[2,3-O-(1-Methylethylidene)-Beta-D-ribofuranosyl]-9H-purine synthesis
- Product Name:2,6-Dichloro-9-[2,3-O-(1-Methylethylidene)-Beta-D-ribofuranosyl]-9H-purine
- CAS Number:52678-40-7
- Molecular formula:C13H14Cl2N4O4
- Molecular Weight:361.18
Yield:52678-40-7 94%
Reaction Conditions:
with toluene-4-sulfonic acid in acetone at 20; for 16 h;
Steps:
1.1 Step 1: ((3aR,4R,6R,6aR)-6-(2,6-dichloro-9H^urin-9-yl)-2,2-dimethyltetrahydrofurof3,4- d][ l,3]dioxol-4-yl)methanol
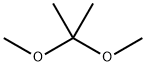
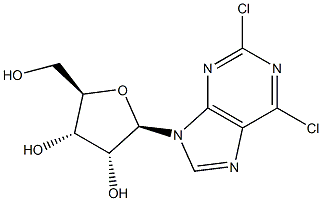
00267] A mixture of (2R,3R,4S,5R)-2-(2,6-dichloro-9H-purin-9-yl)-5-(hydroxymethyl)- tetrahydrofuran-3,4-diol (15.05 g, 46.9 mmol, Chemshuttle, USA, Cat 417), 2,2- dimethoxypropane (60 mL), and /?-toluenesulfonic acid mono hydrate (11.16 g) in acetone (460 mL) was stirred at RT for 16 h. Sodium bicarbonate (15.56 g) and water (300 mL) were added and stirred for 2 h. The resulting mixture was extracted with ethyl acetate (3 x 500 mL). The combined organic phase was dried over Na2S04 and solvents were evaporated under reduced pressure. The residue was purified by chromatography on silica gel (330 g column eluted with 0 → 4% methanol in DCM over 60 min) to afford 15.93 g (94%) of ((3aR,4R,6R,6aR)-6-(2,6- dichloro-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][l,3]dioxol-4-yl)methanol as a solid. LCMS Method 1 : fe = 1.21 min, m/z 361, 363 (MH+).
References:
WO2015/164573,2015,A1 Location in patent:Paragraph 00267