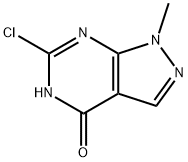
6-Chloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4(7H)-one synthesis
- Product Name:6-Chloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4(7H)-one
- CAS Number:5334-35-0
- Molecular formula:C6H5ClN4O
- Molecular Weight:184.58
![4,6-dichloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/98141-42-5.gif)
98141-42-5
![6-Chloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4(7H)-one](/CAS/20180713/GIF/5334-35-0.gif)
5334-35-0
General procedure for the synthesis of 6-chloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4(7H)-one from 4,6-dichloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidinium: In a sealed reaction vessel, 4,6-dichloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidinium (2.2 g, 10.8 mmol) and 2 M sodium hydroxide aqueous solution (50 mL). After sealing the vessel, the reaction mixture was heated to 70 °C and stirred for 30 min under the protection of a blast shield. Upon completion of the reaction, the clarified reaction solution was diluted with water and the pH was adjusted to 6-7 with 2M aqueous hydrochloric acid solution, followed by vacuum concentration. The precipitated solid was collected by filtration and washed with ethanol (~300 mL). The filtrate was concentrated in vacuum and adsorbed on Celite and purified by fast chromatography (using a 40 g silica gel column with 1-10% methanol:dichloromethane as eluent) to afford 6-chloro-1-methyl-1,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one as a white solid (1.45 g, 72.5% yield). The product was confirmed by 1H NMR (300 MHz, DMSO-d6): δ 3.87 (s, 3H), 8.06 (s, 1H), 13.18 (br.s., 1H).Results of the LC-MS analysis: calculated value C6H6ClN4 [(M+H)+] 185, measured value 184.9.
![4,6-dichloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/98141-42-5.gif)
98141-42-5
98 suppliers
$19.00/100mg
![6-Chloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4(7H)-one](/CAS/20180713/GIF/5334-35-0.gif)
5334-35-0
32 suppliers
$66.00/100mg
Yield:5334-35-0 72.5%
Reaction Conditions:
with sodium hydroxide in water at 70; for 0.5 h;Sealed tube;
Steps:
Intermediate A 6-Chloro-l -methyl- l,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one
Intermediate A 6-Chloro-l -methyl- l,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one A sealed reaction vessel was charged with 4,6-dichloro-l-methyl-lH-pyrazolo[3,4- d]pyrimidine (US 20100015141 Al, 2.2 g, 10.8 mmol) and a 2M aqueous sodium hydroxide solution (50 mL). The vessel was sealed, and the reaction was heated to 70°C behind a blast shield and stirred for 30 min. The resulting clear solution was transferred with water, brought to pH 6-7 with a 2M aqueous hydrochloric solution and concentrated in vacuo. The remaining solids were filtered and rinsed with ethanol (-300 mL), and the filtrate was concentrated in vacuo onto Celite. Flash chromatography (40 g silica gel column, 1-10% methanol: methylene chloride) afforded 6-chloro-l-methyl-l,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one as a white solid (1.45 g, 72.5%). 1H NMR (300 MHz, DMSO-d6) δ ppm 3.87 (s, 3 H) 8.06 (s, 1 H) 13.18 (br. s., 1 H). LC-MS calcd. for C6H6C1N40 [(M+H)+] 185, obsd. 184.9.
References:
WO2013/182546,2013,A1 Location in patent:Page/Page column 85