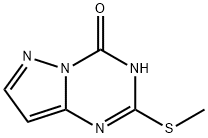
2-(METHYLTHIO)PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4(3H)-ONE synthesis
- Product Name:2-(METHYLTHIO)PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4(3H)-ONE
- CAS Number:54346-18-8
- Molecular formula:C6H6N4OS
- Molecular Weight:182.2
![2-Thioxo-2,3-dihydropyrazolo[1,5-a][1,3,5]triazin-4(1H)-one](/CAS/GIF/34682-99-0.gif)
34682-99-0

74-88-4
![2-(METHYLTHIO)PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4(3H)-ONE](/CAS/GIF/54346-18-8.gif)
54346-18-8
General procedure for the synthesis of 2-(methylthio)pyrazolo[1,5-α][1,3,5]triazin-4(1H)-one from 2-thio-2,3-dihydropyrazolo[1,5-a][1,3,5]triazin-4(1H)-one (7.99 g, 0.05 mol) and iodomethane (3 mL, 0.05 mol): firstly, 2-thio-2,3-dihydro pyrazolo[1,5-a][1,3,5]triazin-4(1H)-one was first suspended in a solvent mixture of ethanol (200 mL) and 2M aqueous sodium hydroxide (48 mL). Since the mixture was too viscous, it needed to be diluted by adding about 10 mL of water. The mixture was then cooled to 0°C and iodomethane was slowly added dropwise. During the dropwise addition, the mixture briefly dissolved and then began to form a white precipitate. The reaction system was gradually warmed to room temperature with continuous stirring for 3 hours, after which it was left overnight. Upon completion of the reaction, it was cooled again to 0 °C, 2N aqueous sulfuric acid solution (58 mL) was added and stirred for 1 hour. The precipitate was collected by filtration, washed sequentially with water (200 mL) and cyclohexane (50 mL), and finally dried in a vacuum oven to afford the target product 2-(methylthio)pyrazolo[1,5-α][1,3,5]triazin-4(3H)-one (6.85 g, 79% yield) as a white solid. The product was confirmed by NMR hydrogen spectroscopy (DMSO-d6): δH 12.94 (s, 1H), 7.96 (d, J=1.9Hz, 1H), 6.34 (d, J=1.9Hz, 1H), 2.53 (s, 3H).
![2-Thioxo-2,3-dihydropyrazolo[1,5-a][1,3,5]triazin-4(1H)-one](/CAS/GIF/34682-99-0.gif)
34682-99-0
38 suppliers
$62.00/100mg

74-88-4
357 suppliers
$15.00/10g
![2-(METHYLTHIO)PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4(3H)-ONE](/CAS/GIF/54346-18-8.gif)
54346-18-8
75 suppliers
$45.00/10mg
Yield:54346-18-8 92.3%
Reaction Conditions:
with sodium hydroxide in ethanol;water monomer at 15; for 1 h;
Steps:
111.3 Step 3 : 2-Methylsulfanyl-3H-pyrazolo[1,5-a][1,3,5]triazin-4-one
To a suspension of 2-thioxo-lH-pyrazolo[l,5-a][l,3,5]triazin-4-one (16 g, 90.38 mmol, 1 eq) in EtOH (120 mL) was added NaOH (7.23 g, 180.76 mmol, 2 eq) in H20 (9.6 mL), Mel (15.96 g, 112.44 mmol, 7.0 mL, 1.24 eq) was added dropwise. The mixture was stirred at 15 °C for 1 h. The mixture was acidified with IN aq. HC1 (80 mL) and EtOH was evaporated. The mixture was filtered, washed with H20 (50 mL x 2). The filter cake was dried to yield 2-methylsulfanyl-3H- pyrazolo[l,5-a][l,3,5]triazin-4-one (16 g, 83.42 mmol, 92.3% yield, 95% purity) as a white solid. NMR (400 MHz, CD3OD) δ ppm 7.74 (d, J= 2.0 Hz, 1H), 5.99 (d, J = 2.2 Hz, 1H), 2.48 (s, 3H); ES-LCMS m/z 183.2 [M+H]+.
References:
WO2018/195397,2018,A2 Location in patent:Paragraph 00733; 00736
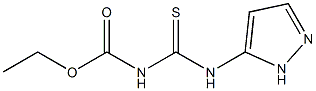
34683-00-6
1 suppliers
inquiry
![2-(METHYLTHIO)PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4(3H)-ONE](/CAS/GIF/54346-18-8.gif)
54346-18-8
75 suppliers
$45.00/10mg

34683-00-6
1 suppliers
inquiry
![2-(METHYLTHIO)PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4(3H)-ONE](/CAS/GIF/54346-18-8.gif)
54346-18-8
75 suppliers
$45.00/10mg