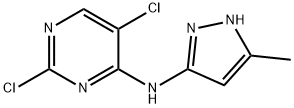
2,5-Dichloro-N-(5-methyl-1H-pyrazol-3-yl)-4-pyrimidinamine synthesis
- Product Name:2,5-Dichloro-N-(5-methyl-1H-pyrazol-3-yl)-4-pyrimidinamine
- CAS Number:543712-81-8
- Molecular formula:C8H7Cl2N5
- Molecular Weight:244.08
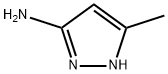
31230-17-8
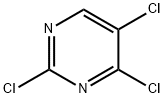
5750-76-5

543712-81-8
The general procedure for the synthesis of 2,5-dichloro-N-(5-methyl-1H-pyrazol-3-yl)-4-aminopyrimidine from 3-amino-5-methyl-1H-pyrazole and 2,4,5-trichloropyrimidine was as follows: to a solution of 5-methyl-1H-pyrazol-3-amine (4.00 g, 41.2 mmol) in anhydrous ethanol (100 mL) was added triethylamine (12.51 g (123.6 mmol) and 2,4,5-trichloropyrimidine (7.56 g, 41.2 mmol). The reaction mixture was stirred at room temperature for 16 h and subsequently concentrated under vacuum. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate, v/v = 1/1) to afford the target compound 2,5-dichloro-N-(5-methyl-1H-pyrazol-3-yl)-4-aminopyrimidine as a light yellow solid (9.00 g, 89.5% yield). Mass spectrum (ESI, positive ion mode) m/z: 244.1 [M + H]+; NMR hydrogen spectrum (600 MHz, DMSO-d6) δ (ppm): 12.32 (broad single peak, 1H), 9.70 (single peak, 1H), 8.33 (single peak, 1H), 6.28 (single peak, 1H), 2.25 (single peak, 3H).

31230-17-8
368 suppliers
$5.00/5g

5750-76-5
368 suppliers
$5.00/1g

543712-81-8
35 suppliers
$13.00/100mg
Yield:543712-81-8 89.5%
Reaction Conditions:
with triethylamine in ethanol at 20; for 16 h;
Steps:
1.6 Step 6) 2,5-dichloro-Af-(5-methyl-l /-pyrazol-3-yl)pyrimidin-4-amine
To a solution of 5-methyl-l /-pyrazol-3-amine (4.00 g, 41.2 mmol) in absolute EtOH (100 mL) were added Et3 (12.51 g, 123.6 mmol) and 2,4,5-trichloropyrimidine (7.56 g, 41.2 mmol). The reaction was stirred at room temperature for 16 h and concentrated in vacuo. The residue was purified by silica gel column chromatography (PE/EtOAc (v/v) = 1/1) to give the title compound as a pale yellow solid (9.00 g, 89.5%). MS (ESI, pos. ion) m/z: 244.1 [M+H]+; NM (600 MHz, DMSO-i: δ (ppm) 12.32 (br. s, 1H), 9.70 (s, 1H), 8.33 (s, 1H), 6.28 (s, 1H), 2.25 (s, 3H).
References:
WO2015/94803,2015,A1 Location in patent:Paragraph 323