4-Pyrimidinamine, 2-chloro-N-(5-methyl-1H-pyrazol-3-yl)- synthesis
- Product Name:4-Pyrimidinamine, 2-chloro-N-(5-methyl-1H-pyrazol-3-yl)-
- CAS Number:543712-91-0
- Molecular formula:C8H8ClN5
- Molecular Weight:209.6356
Yield:543712-91-0 90%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dimethyl sulfoxide at 60; for 16 h;
Steps:
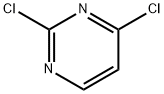
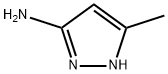
8.1 Step-1: Synthesis of compound J - 2-chloro-N-(5-methyl-1H-pyrazol-3-yl)pyrimidin-4-aniine
A mixture of 2,4-dichloropyrimidine (0.5 g, 3.3 mmol), 3-chloro-5-methyl-1H-pyrazole (0.355 g, 3.6 mmol) and DIPEA (0.6 mL, 3.3 mmol) in DMSO (5 mL) were stirred at 60 °C for 16 h. The reaction was monitored by TLC, starting material was consumed. The reaction mixture was cooled to room temperature, added water and solid was precipitated. The solid was filtered, washed with pet ether and dried under vacuum to yield compound J 2-chloro-N-(5-methyl-1H-pyrazol-3-yl)pyrimidin-4-amine (0.596 g, 90 % yield). LC purity: 96.69%; m/z: 210.2 [M+H]+ (Mol. formula C8HSC1N5, calcd. mol. wt. 209.64).
References:
WO2020/172258,2020,A1 Location in patent:Paragraph 00137