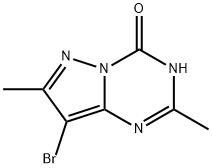
8-BroMo-2,7-diMethyl-3H-pyrazolo[1,5-a][1,3,5]triazin-4-one synthesis
- Product Name:8-BroMo-2,7-diMethyl-3H-pyrazolo[1,5-a][1,3,5]triazin-4-one
- CAS Number:55457-59-5
- Molecular formula:C7H7BrN4O
- Molecular Weight:243.06
![2,7-DIMETHYL-3H,4H-PYRAZOLO[1,5-A][1,3,5]TRIAZIN-4-ONE](/CAS/20180529/GIF/55785-87-0.gif)
55785-87-0
7 suppliers
inquiry
![8-BroMo-2,7-diMethyl-3H-pyrazolo[1,5-a][1,3,5]triazin-4-one](/CAS/GIF/55457-59-5.gif)
55457-59-5
16 suppliers
$295.00/0.25G
Yield:55457-59-5 82%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 0 - 20; for 3.16667 h;
Steps:
8-Bromo-2,7-dimethylpyrazolo[1,5-a][1,3,5]triazin-4(3H)-one
2,7-Dimethylpyrazolo[1,5-a][1,3,5]triazin-4(3H)-one (616 mg, 3.75 mmol) was added to CH2Cl2 (25 mL) at 0 °C. Cold N-bromosuccinimide (801 mg, 4.50 mmol) was added and the reaction mixture was stirred at 0 °C for 10 minutes. The reaction mixture was gradually brought to RT and further stirred for 3 h. White solid precipitated out of solution and the compound was collected via vacuum filtration to give 8-bromo-2,7-dimethylpyrazolo[1,5-a][1,3,5]triazin-4(3H)-one (744 mg, 3.1 mmol, 82% yield). 1H NMR (400 MHz, DMSO-d6) δ = 12.53 (br s, 1H), 2.34 (s, 3H), 2.30 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ = 156.2, 153.4, 146.9, 143.8, 85.9, 21.3, 13.3. LCMS (Formic) 93% desired product; tret = 0.60 min, MH+ 243.0.
References:
Cosgrove, Brett;Down, Kenneth;Bertrand, Sophie;Tomkinson, Nicholas C.O.;Barker, Michael D. [Bioorganic and Medicinal Chemistry Letters,2021,vol. 33,art. no. 127752] Location in patent:supporting information
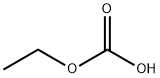
13932-53-1
4 suppliers
inquiry

1304777-34-1
10 suppliers
$45.00/10mg
![8-BroMo-2,7-diMethyl-3H-pyrazolo[1,5-a][1,3,5]triazin-4-one](/CAS/GIF/55457-59-5.gif)
55457-59-5
16 suppliers
$295.00/0.25G