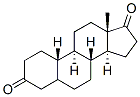
(5R,8R,9R,10S,13S,14S)-13-Methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione synthesis
- Product Name:(5R,8R,9R,10S,13S,14S)-13-Methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione
- CAS Number:5696-51-5
- Molecular formula:C18H26O2
- Molecular Weight:274.4
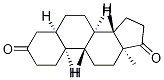
734-32-7
226 suppliers
$300.00/1g
![(5R,8R,9R,10S,13S,14S)-13-Methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione](/CAS/GIF/5696-51-5.gif)
5696-51-5
18 suppliers
inquiry
Yield:5696-51-5 89%
Reaction Conditions:
with 5%-palladium/activated carbon;hydrogen bromide;hydrogen in tetrahydrofuran at 20; under 3620.13 Torr; for 24 h;
Steps:
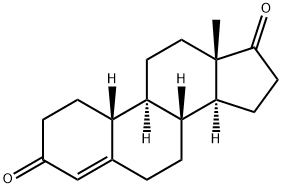
1.1 [445] Step 1 : Synthesis of (5R,8R,9R, 10S, 13S,14S)-13-methyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,17(2H)-dione (Compound 2)
A mixture of compound 1 (50.0 g, 184.56 mmol, 1.0 eq), palladium black (2.5 g, 5% w/w) in tetrahydrofuran (300 mL) and concentrated hydrobromic acid (1 mL) was hydrogenated with 70 psi hydrogen. The mixture was stirring at room temperature for 24 h. The progress of the reaction mixture was monitored by TLC. After completion of the reaction, the mixture was filtered through a pad of celite and sintered funnel and the filtrate was concentrated under reduced pressure. The residue was recrystallized from acetone to afford compound 2 (45.0 g, 89%). TLC: hexane / ethyl acetate (3: 1); Rf: (Compound 1) = 0.4; Rf: (Compound 2) = 0.3.
References:
WO2020/150210,2020,A1 Location in patent:Paragraph 443; 445-446

19468-31-6
4 suppliers
inquiry
![(5R,8R,9R,10S,13S,14S)-13-Methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione](/CAS/GIF/5696-51-5.gif)
5696-51-5
18 suppliers
inquiry

10002-97-8
4 suppliers
inquiry
![(5R,8R,9R,10S,13S,14S)-13-Methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione](/CAS/GIF/5696-51-5.gif)
5696-51-5
18 suppliers
inquiry
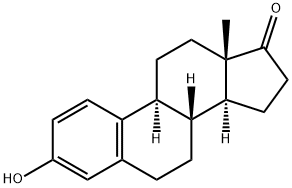
53-16-7
587 suppliers
$10.00/1g
![(5R,8R,9R,10S,13S,14S)-13-Methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione](/CAS/GIF/5696-51-5.gif)
5696-51-5
18 suppliers
inquiry