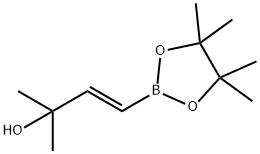
(E)-2-Methyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)but-3-en-2-ol synthesis
- Product Name:(E)-2-Methyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)but-3-en-2-ol
- CAS Number:581802-26-8
- Molecular formula:C11H21BO3
- Molecular Weight:212.09
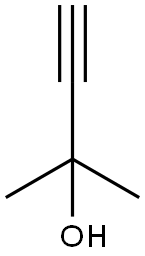
115-19-5
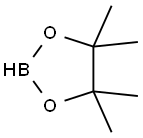
25015-63-8

581802-26-8
GENERAL METHOD: Tris(2-furyl)phosphine (0.02 mmol, 4.7 mg) was mixed with [Rh(CO)2Cl]2 (0.01 mmol, 3.9 mg) in toluene (10 mL) at room temperature. After stirring for 10 min, a solution of 2-methyl-3-butyn-2-ol (1 mmol) in toluene (1 mL) and pinacolborane (1.2 mmol, 174 μL) were added sequentially to the reaction system. The reaction was stirred at room temperature under nitrogen protection and the progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction was quenched with water and the reaction mixture was subsequently extracted with ether (Et2O). The organic phases were combined, dried with anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure to remove the solvent. Finally, the residue was purified by silica gel column chromatography to afford the target product (E)-2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3-buten-2-ol.

115-19-5
264 suppliers
$20.00/25mL

73183-34-3
587 suppliers
$6.00/5g

581802-26-8
44 suppliers
$45.00/10mg
Yield:581802-26-8 97%
Reaction Conditions:
with sodium methylate;Cu(2+)*4Cu(1+)*4I(1-)*2C5H3N2O2(1-)*C5H5N*C2H7N*3C3H7NO in ethanol at 25; for 1 h;Inert atmosphere;
Steps:
2.3. Typical procedure for the hydroborylation of alkynes
General procedure: The typical experimental procedure for the hydroborylation ofalkynes is as follows: a mixture of alkyne (2 mmol), compound 1(0.006 mmol), bis(pinacolato)diboron (2.5 mmol), NaOMe(0.2 mmol) and 5 mL EtOH were successively added into a reaction flask equipped with a Ar (99.99%) balloon. Then the flask was vacuumedand charged in Ar three times to exclude air in the reactionsystem. Subsequently, the obtained reaction mixture was magneticallystirred at 25 °C for 1 h. After the reaction was completed, thecatalysts were separated by filtration and the yield was determinedby GC analysis. The pure products were obtained by columnchromatography on silica gel (petroleum ether/ethyl acetate as aneluent).
References:
Gao, Ning;Hu, Tianding;Kang, Xiaomin;Lan, Xingwang;Wang, Zhenguang;Wu, Zhi-Lei;Zhao, Bin [Journal of Catalysis,2021,vol. 404,p. 250 - 257]

115-19-5
264 suppliers
$20.00/25mL

25015-63-8
216 suppliers
$22.39/5G

581802-26-8
44 suppliers
$45.00/10mg
![1,3,2-Dioxaborolane, 4,4,5,5-tetramethyl-2-[(1E)-3-methyl-3-[(trimethylsilyl)oxy]-1-buten-1-yl]-](/CAS/20200611/GIF/1138162-60-3.gif)
1138162-60-3
1 suppliers
inquiry

581802-26-8
44 suppliers
$45.00/10mg
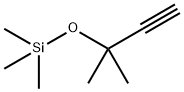
17869-77-1
52 suppliers
$64.50/5ml

25015-63-8
216 suppliers
$22.39/5G

581802-26-8
44 suppliers
$45.00/10mg
558-30-5
245 suppliers
$20.00/5G
![Bis[(pinacolato)boryl]Methane](/CAS/GIF/78782-17-9.gif)
78782-17-9
110 suppliers
$22.00/1g

581802-26-8
44 suppliers
$45.00/10mg